Milk Analysis
Objectives
In this lab you will separate some of the primary components of milk. You will then be able to determine the percent composition of milk in terms of protein, fat, carbohydrates, and phosphate.
BACKGROUND
Milk is a colloidal aqueous suspension consisting of many components, several of which include carbohydrates (sugars), lipids (fats), proteins, and phosphate. The percentage of each of these components will depend upon the source of the milk (e.g., cow, goat, etc.) as well as the methods used to process the milk. In this experiment, you will isolate the fats, proteins, phosphate (PO43-), and carbohydrates from a sample of milk. In addition, you will determine the percentage of fat, protein, and phosphate in your sample of milk.
Protein
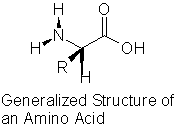
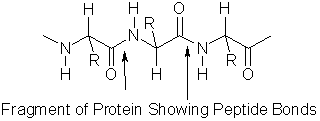
Proteins are large, polymeric structures made up of amino acids. An amino acid is a generic name for one of twenty different molecules. The generalized structure of an amino acid is shown in Figure 1. Each amino acid has a carboxylic acid group on one end of the molecule and an amine group on the other end. R represents a functional group (e.g., -CH3, -CH2CH3, -OH) that is unique to each amino acid. When two amino acids react, a peptide bond is formed between the carboxylic acid group of one amino acid and the amine group of a second amino acid. A protein will contain numerous peptide bonds; it is not uncommon for the molecular weight of a protein to exceed 100,000.
Figure 1. General Protein Structure.


When placed in an aqueous solution, protein will fold up on itself to form what is called a "tertiary" structure. This folding results from the interaction between water molecules and the functional groups on the protein. The polar (hydrophilic or "water loving") R groups lie on the outside of the protein in contact with water while the nonpolar groups (hydrophobic or "water fearing") R groups bury themselves on the inside of the protein. Some molecules, such as acetic acid, can interfere with this process by penetrating the hydrophobic interior of the protein, causing it to unwrap. When the hydrophobic groups come in contact with the water, the protein’s solubility decreases and it precipitates from the solution. This process is referred to as "denaturing" the protein.
Fats
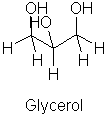
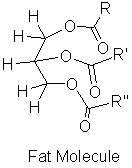
Fats are a type of lipid composed of a tryhydroxy alcohol (glycerol) and long chain fatty acids (RCOOH) as shown in Figure 2.
Figure 2. The glycerol on the left is the central part of the fat molecule on the right.
As in the case of the proteins, the R groups determine the identity and properties of the particular fat. Plant oils, for instance, have a large portion of unsaturated fatty acids (double bonds between many of their carbon atoms).
Oleic acid: CH3(CH2)7CH=CH(CH2)7COOH
Linoleic acid: CH3(CH2)4(CH=CHCH2)2(CH2)6COOH
Animal fats, on the other hand, contain a greater portion of saturated fatty acids (single bonds between carbon atoms).
Palmitic acid: CH3(CH2)14COOH
Steric acid: CH3(CH2)16COOH
Because of the large hydrocarbon part of the fatty acid, the bulk of the fat molecule is hydrophobic. This is why fats have a very low solubility in water but a very large solubility in less polar organic solvents. For this reason, the fat molecules will adhere to the protein with acetone (an organic solvent).
Figure 3. Reaction of a fat molecule to form acrolein.
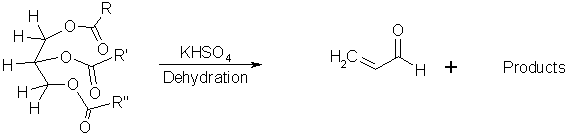
Carbohydrates
Saccharides (sugars) are carbohydrates that have the general formula Cn(H2O)m. Lactose is the main carbohydrate in milk and is a disaccharide containing the monosaccharides glucose and galactose:
Figure 4. Hydrolysis of a disaccharide.
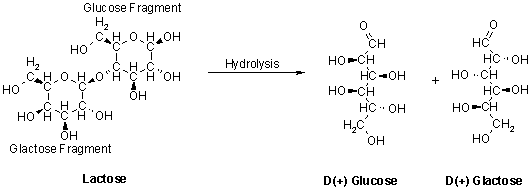
Note the large number of hydroxy (-OH) groups on the molecule. These hydroxy groups make sugars very soluble in aqueous solutions allowing lactose to remain in solution while protein and fat are precipitated out.
 To test for the presence of lactose, you
will take advantage of its ability to reduce Cu2+ to Cu2O in
alkaline solutions forming a rust colored precipitation product. Benedict’s Tests
uses a Cu2+-citrate complex (citrate prevents Cu(OH)2(s) from
forming in the alkaline solution) to oxidize the aldehyde group to a carboxylic acid (see
below). This test gives a positive result for aldehydic sugars (found in lactose), but not
for ketone sugars such as fructose.
To test for the presence of lactose, you
will take advantage of its ability to reduce Cu2+ to Cu2O in
alkaline solutions forming a rust colored precipitation product. Benedict’s Tests
uses a Cu2+-citrate complex (citrate prevents Cu(OH)2(s) from
forming in the alkaline solution) to oxidize the aldehyde group to a carboxylic acid (see
below). This test gives a positive result for aldehydic sugars (found in lactose), but not
for ketone sugars such as fructose.
Phosphate
The last component you will isolate is the phosphate ion, PO43-. You will do this by adding Ca2+ which precipitates the phosphate in the form of the insoluble salt, Ca3(PO4)2.
DATA COLLECTION
You should work in pairs. Student A will isolate the protein and fat (this part is labeled "Student A" in the flow chart). Student B will isolate the phosphate and lactose (labeled "Student B" in the flow chart). Record all observations in your lab notebook.
This experiment is designed to require two laboratory periods
Students A and B
Label the following items:
1-250 mL beaker – "fat"
2-weighing boats – "protein" and "Ca3(PO4)2"
Weigh these labeled containers and record the data in your notebook. These containers will be used to store your isolated products until the next lab period. Keep them in your drawer until you are ready to use them.
Place a clean, dry 150 mL beaker on the top loading balance. Tare the balance and add about 50 mL of milk to the beaker. Record the exact mass.
Add ~1 mL of 8 M acetic acid to the milk and stir while heating on your hot plate (at a medium setting) until the mixture almost boils. The protein and adhering fat should completely precipitate in about 5 minutes.
Cool this mixture thoroughly (~10 minutes) in an ice water bath (use a large beaker to make your ice water bath). While the mixture is cooling, take care not to disturb its contents.
To filter out the protein and fat, place the center of a clean cheesecloth loosely over a 250 mL beaker and pour the mixture through the cloth into the beaker. Gently squeeze the cheesecloth containing your precipitate to allow the excess liquid to drain into the beaker. Remove the precipitate from the cheesecloth and place it in a clean 150 mL beaker.
At this point, Student A should take the precipitate and proceed with the separation of the protein and fat. Student B should use the filtrate to remove the phosphate from the lactose.
Student A: Protein and Fat
To remove the adhering fat from the protein (casein), add 35 mL of acetone to the precipitate. Break the solid into small pieces with your stirring rod and place the beaker inside your hood on your hot plate. With a moderately fast stirring rate (to avoid "bumping" your solution) SLOWLY heat your mixture on the lowest setting. Because acetone evaporates quickly, you should periodically replenish the acetone to its original volume.
When the solution comes to a boil, remove the beaker from the hot plate to a paper towel on your lab bench (still under the hood). After allowing it to cool for about 2 minutes, reheat just to boiling and cool as before, repeating this process for a total of 3 times. This should be sufficient to remove the fat.
Filter the casein using your Büchner funnel and wash with a small amount (~5 mL) of acetone. Continue drawing air through the casein until it is dry and easily removed from the filter paper (about 5 minutes). Place the casein in the weighing boat labeled "protein" and carefully place it in your lab drawer to dry until the next lab period.
Transfer the filtrate into the beaker labeled "fat". Try to remove as much fat from the flask as possible by rinsing twice with ~3 mL acetone. Add this to the filtrate in the beaker. Carefully place the beaker in your lab drawer. During the week all, or most, of the acetone will evaporate.
Student B: Phosphate and Lactose
To the filtrate from the first part (containing phosphate and lactose) add ~7 mL of 1 M NaOH. Stir the solution and test with litmus paper to make sure it is basic. If it is not basic, add an additional mL of NaOH until it becomes basic.
Add 10 mL of 0.5 M Ca(NO3)2 and heat on a medium setting. While stirring with your magnetic stir bar, continue heating until the volume has been reduced by about one-third (~15 minutes). Allow the solution to cool undisturbed in an ice bath for about 5 minutes.
Filter the solid Ca3(PO4)2 using a Büchner funnel. Wash with two 2 mL portions of cold water, two 2 mL portions of ethanol, and one 5 mL portion of acetone. Dry the precipitate until it is easily removed from the filter paper (~5 minutes) and scrape it into the weighing boat labeled "Ca3(PO4)2".
Use the filtrate (containing lactose) to perform the class test for reducing sugars.
Benedict’s Test for Reducing Sugars
Set up a boiling water bath on your hot plate and prepare two test tubes. To both test tubes, add 5 mL of Benedict’s solution. To the first test tube, add 8 drops of the prepared 1% glucose solution. Glucose (a reducing sugar) will give a positive test result and will be used to compare with the results obtained from your lactose solution. To the second test tube, add 8 drops of your lactose solution. Mix the contents of both test tubes well and place them in the boiling water bath for 3-5 minutes. Note the results in both test tubes.
On Week two, CALCULATE THE PERCENT COMPOSITION AND SHARE YOUR RESULTS WITH YOUR CLASSMATES BEFORE LEAVING THE LABORATORY.
REPORT
Prepare a short report (1-2 pages) using the outline below. The report should be grammatically correct. Avoid wordiness and attempt to clearly state your point (brevity is better, but you must write enough information to indicate that you understand.
I. Introduction
Briefly (three to five sentences) describe the experiment. What were the major concepts? Which techniques need to be included in the introduction?
Things to Think About?
A general solubility rule is "like dissolves like."
How will you draw large molecules such as a protein?
What features of a protein might be related to solubility?
Include data pages, sample calculations, tables, and graphs as part of the appendices. Each member of the group should clearly indicate their contribution to the data collection process.