Background:
A soap is the sodium or potassium salt of a long chain fatty acid. The fatty acid usually contains 12 to 18 carbon atoms. Solid soaps usually consist of sodium salts of fatty acids whereas liquid soaps usually are potassium salts of fatty acids. A soap, such as the sodium stearate you will be preparing in this experiment, consists of a polar end and a non-polar end.
O
| |
CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2-C-O-Na+
nonpolar polar
(dissolves in oils) (dissolves in water)
Because like dissolves in like, the non-polar end (hydrophobic or water-fearing part) of the soap molecule can dissolve the greasy dirt, and the polar or ionic end (hydrophilic or water-loving part) of the molecule is attracted to water molecules. Therefore the dirt from the surface being cleaned will be pulled away and suspended in water. Thus soap acts as an emulsifying agent, a substance used to disperse one liquid (oil molecules) in the form of finely suspended particles or droplets in another liquid (water molecules).
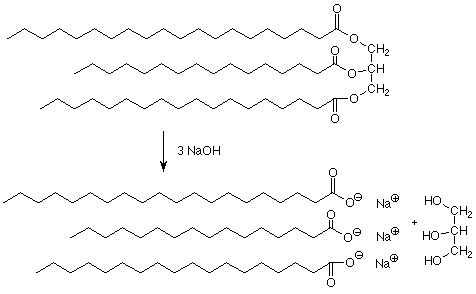
Treatment of fats or oils with strong bases such as lye (NaOH) or potash (KOH) causes them to undergo hydrolysis (saponification) to form glycerol and the salt of a long-chain fatty acid (soap).
|
Because soaps are the salts of strong bases and weak acids they should be slightly basic. If a soap is too basic it could cause damage to skin, surfaces to be cleaned, or clothes. We will test the basicity/ acidity of the soap you create.
Since the cleansing action of soaps depends upon the fact that they ionize readily in water, you can imagine what would happen if the ionic end lost its charge. The soap would no longer be attracted to water molecules and could no longer emulsify oil and dirt. This is just what happens in hard or acidic water. Hard water contains metal cations, such as Ca2+ and Mg2+, that react with the charged ends of the soaps to form insoluble salts. The insoluble salts that Ca2+ and Mg2+ form with soap anions cause the gray precipitate commonly called bathtub ring.
Synthetic detergents were developed to overcome these limitations of soaps. Detergents are similar to soaps in having an ionic end and a nonpolar end. They have different structures, however, which make them less susceptible to forming insoluble Ca2+ and Mg2+ salts. Many detergents and a few soaps contain phosphates, which serve as bases to neutralize acidic water and also to form insoluble salts with the Ca2+ and Mg2+ ions. This prevents the reaction with soap that forms bathtub ring. However, soluble phosphate salts, like sodium phosphate, when released into rivers and lakes can cause explosive growths of algae. This can cause decay or eventual death of the aquatic ecosystem due to deoxygenation from the decomposition of dead algae. Because of this, phosphates in detergents have been outlawed in many places. See the following web site for more information about this.
In this experiment you will make a soap using a vegetable oil as your starting material. Then, you will compare the properties of the soap you made with those of a commercial soap and a commercial detergent.
Purpose: To synthesize a soap and compare its properties with the properties of a commercial soap and detergent.
Wastes: All of the chemicals from this experiment can be disposed of down the drain or in the trash. The water should be running before disposal of excess NaOH solution.
Be careful with all glassware, they get very slippery when soapy and wet!
Procedure:Preparation of the Sodium Soap: Measure 12mL of vegetable oil or shortening into a 400mL beaker. Add 10 ml of ethanol. The ethanol and oil will separate into layers. Add 12mL of 5M NaOH. Stir the mixture vigorously with a glass rod and gently heat on a hot plate for 15 minutes or until it turns pasty.
When the paste begins to form, stir very carefully to prevent frothing!
The paste is a mixture of soap and glycerol. While it is heating, observe the solution. How many layers are there? Why do you think this is true? Do you observe any smells coming off your solution? What is the identity of the smell? How do you know?
After all the paste has formed, set the beaker on your bench to cool. When the paste mixture is cool, add 50mL of saturated NaCl to the paste mixture and stir in thoroughly, breaking up the chunks as you stir. The NaCl solution provides Na+ and Cl- ions that bind to the polar water molecules, and help separate the water from the soap. This process is called salting out the soap.
After stirring the NaCl solution through the soap paste, filter off the soap mixture by suction filtration (see picture and movies) and wash the collected soap precipitate with 15mL of ice water. Continue the suction for an additional 10 minutes to help dry the soap.
Testing the Properties:
First, make separate test solutions of your prepared soap, a commercial soap, and a commercial detergent by dissolving about 1g of each in 100mL of deionized water.
pH: Test the pH of each test solution by touching a clean glass rod to the solution and transferring a drop to a piece of both red and blue litmus paper. Record your results.
Cleaning Ability: Test the different solutions ability to clean fat off a piece of glassware. Spread solid vegetable shortening on watch glasses and test the ability of solutions of your soaps to wash this off. Compare your soap results with the commercial soap, commercial detergent, and deionized water and record your results.
Behavior in acidic water: Hydronium ions attach to one end of soap molecules, destroying their cleaning ability by reducing their attraction for water molecules. (Which end of the soap molecule will hydronium ions be attracted to?) To test this, place 10mL of each of your test solutions and distilled water in clean separate test tubes, add 5 drops 3M HCl to each test tube, shake well, and record your observations. Next add 10 drops of oil to each test tube and shake well. Note your observations immediately on the recording sheet, observe again after the solutions have sat for 5 minutes. What conclusions can you make about the different cleaning solutions?
Behavior in Hard Water: Before you begin the next part, put a 400mL beaker half full of water on a hot plate to heat up. In this part, you will test the effects of hard water on soap’s ability to act. Obtain about 10mL of hard water in a clean beaker. Place 10mL of each of your 3 test solutions into separate clean labeled test tubes. Add 2mL of the hard water solution to each test tube. After each addition, shake well and immediately observe the nature of the contents, looking for cloudiness, precipitates, films, etc. Record your observations. Allow for the test tubes to stand undisturbed for 5 minutes, observe their condition now and record these observations also. Repeat the procedure using distilled water in place of the hard water solution.
Phosphates: To test for phosphates in your test solutions and deionized water,
place 2mL of each solution into clean separate test tubes. Add 5 drops of 1M HNO3
and 2mL of 1% ammonium molybdate solution to each test tube. Warm the test tubes in a
water bath but do not boil the solutions. A yellow solution or formation of a precipitate
indicates the presence of phosphate.
Record your results.
Go to Prelab Questions, Data Sheet, and Post-lab Questions (note: the pre-lab link is separate from the data sheet and post-lab link)