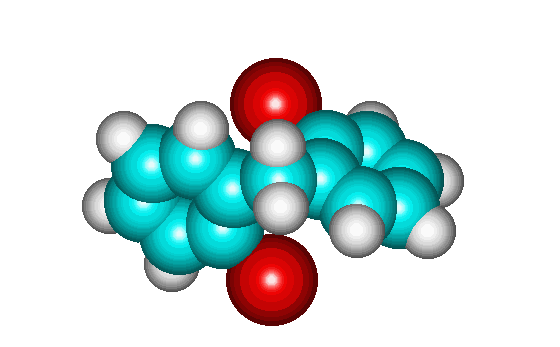

Space-filling representation of geometry-optimized 2,2'diiiododiphenylmethane as rendered by HyperChem Lite molecular modeling software. Notice that the molecule is chiral (no planes of symmetry are present) even though it possesses no chiral centers and single bond rotations would convert it to its enantiomer. In this case the bond rotation is prevented by steric interactions - the iodines are too large to rotate past the hydrogen of the other ring.