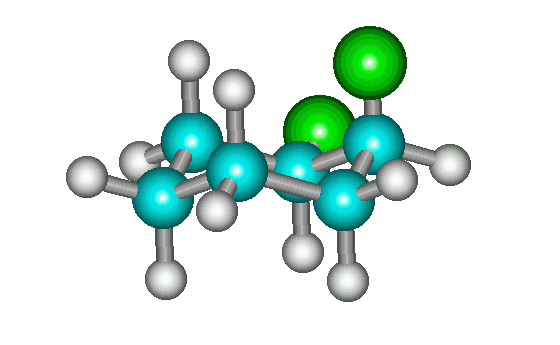
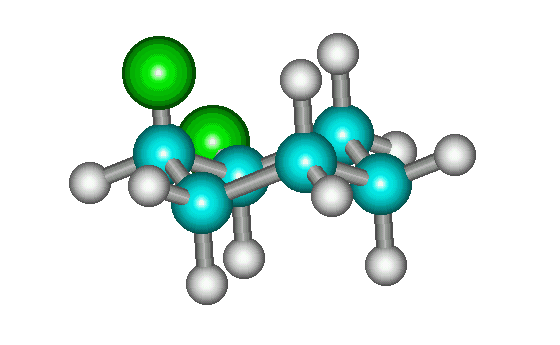
cis-1,2-dichlorocyclohexane


The most stable conformation of cis-1,2-disubstituted cyclohexanes with two identical groups lacks a plane of symmetry and, therefore, would appear to be chiral (see enantiomers above). However, all it takes is a ring flip to interconvert the two enantiomers (see below). Therefore, the molecule for all practical purposes can be considered to be achiral. (Technically, it exists as a pair of conformational enantiomers.) Interactive version is here.

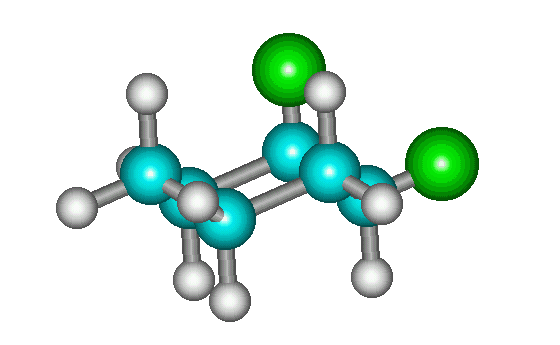
The ring flip of the molecule at left is shown at right. The ring-flipped molecule is identical to the mirror image shown at right at the top of the page.