|
Chemistry 350 Laboratory - Fall Semester 2010 – Professor T. Nalli, Winona
State University
Expt
#4.
Resolution of a Racemic Amine
Relevant textbook readings - Mohrig, Chapter
8 (especially 8.2-8.4), Chapter 14.
Overview - You will isolate the levorotatory enantiomer from a
sample of racemic α-phenylethylamine.
This will be accomplished through fractional crystallization of the (+)-tartrate salt of the (−)-amine.
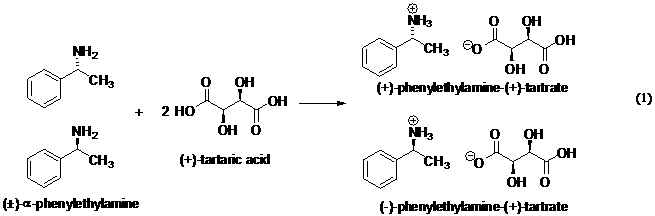
Background - Tartaric acid is a
natural product that occurs naturally as the (+) enantiomer. It will be used
as a resolving agent for racemic α-phenylethylamine,
which it reacts with in acid/base fashion (eq 1). This reaction forms diasteromeric salts, (+)-amine (+)-tartrate
and (−)-amine (+)-tartrate.
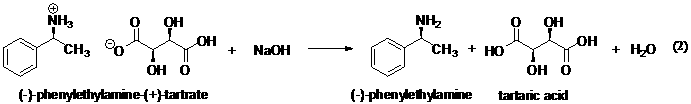
The (−)-amine (+)-tartrate
is less soluble than the other salt and can be crystallized out of methanol
solution. The obtained crystals are then converted back to (−)-α-phenylethylamine by reaction with aqueous sodium
hydroxide (eq 2).
You will use both polarimetry and NMR to measure
the enantiomeric excess of your final product. Ideally, the obtained final
product would be enantiomerically pure (−)-α-phenylethylamine (ee = 100%).
However, in practice, resolution of racemic mixtures rarely gives such a
result. Thus, you can expect an ee of somewhat less
than 100%.
Procedures
Running the reaction. Weigh
out 7.5 g of (+)-tartaric acid and add it to a 250-mL Erlenmeyer flask. Add 120
mL methanol and heat on a hot plate to about 60°C. To the hot solution, slowly
add 6.4 mL of α-phenylethylamine. Stopper the flask, label it thoroughly,
and let it stand in the designated area in the lab until next week.
Week 2 – Check your flask for crystals. If you have no crystals then
either borrow a crystal from another group and add it to your flask as a seed
crystal or use a glass rod to scratch the inside of the flask to induce
crystal growth. For best results the crystals that form should be prismatic
rather than needle-like. If your crystals appear to be mostly needles with
some prisms present, you may want to heat until most of the solid is dissolved and then cooling slowly. (The
needles should dissolve quickly leaving behind a few prismatic crystals,
which can then act as seeds for the growth of more prisms.)
Work-up procedures.
·
Collect
the crystals by vacuum filtration on a pre-weighed Buchner funnel and wash
them with two 5-mL portions of ice cold methanol.
·
Weigh
the funnel with crystals and calculate the yield and percent yield of (−)-α-phenylethylamine tartrate.
·
Add
the crystals to 25 mL H2O in a 100-mL Erlenmeyer and add 4 mL 50% NaOH(aq). Swirl to mix the contents and note your
observations.
·
Transfer
the solution to a separatory funnel (Chapter 8) and extract with 10-mL
dichloromethane (DCM). Drain the lower, organic layer to a clean dry flask
labeled “DCM extracts”. Extract the aqueous solution in the separatory funnel
with an additional 10-mL DCM and again drain the lower layer into the labeled
flask. Repeat this process once more. Discard the remaining aqueous layer.
·
Add
about 1 g anhydrous Na2SO4 to the DCM extracts and
allow the solution to dry for at least ten minutes. (See Chapter 8.8-8.11).
·
Transfer
the dried solution to a small pre-weighed round-bottom flask and heat gently
with a hot water bath to evaporate the solvent. Use a stream of nitrogen from
a pipet blown on the surface of the liquid to
expedite the evaporation process. Once the volume of liquid is down to about 2-3
mL then discontinue this process and place the flask under vacuum for a few
minutes to remove remaining solvent. (Heating the amine in the absence of
solvent is likely to promote the reaction of carbon dioxide in the air with
it. This reaction forms a white solid, which interferes with the polarimetry measurements, so we are attempting to avoid
it by not evaporating all of the solvent with heat.)
·
Weigh
the flask with amine product and calculate the yield and percent yield of (−)-α-phenylethylamine.
Determination of enantiomeric
excess (ee).
1H NMR
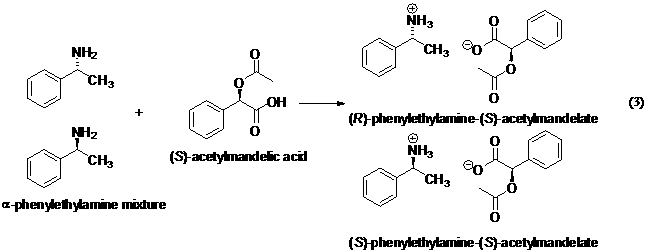
Method. The two enantiomers of α-phenylethylamine
have identical NMR spectra as do any pair of
enantiomers. Therefore, a chiral resolving agent must be used to allow the
detection and quantitation of each compound
separately. We will use (S)-acetylmandelic acid
(AMA) as the chiral resolving agent. This is very simply accomplished by
adding AMA to the NMR tube along with the amine and the NMR solvent (see
procedures below). An acid/base reaction occurs to form diasteromeric
salts (eq 3). These salts have somewhat different 1H NMR spectra,
so NMR can be used to estimate the relative amount of each and, thus, the ee.
·
Place
5 mg of the product into a small test tube. Keep the test tube corked as much
as possible to prevent reaction of the amine with carbon dioxide in the
atmosphere.
·
Weigh
out 10 mg of (S)-O-acetylmandelic acid and add it
to the test tube.
·
Add
about 0.25 mL CDCl3 and mix the solution so as to dissolve the
contents completely.
·
Add
the mixture to an NMR tube and add enough CDCl3 to bring the level
of liquid up to that necessary for good shimming (~ 5
cm).
·
Obtain
the 1H NMR spectrum.
·
Integrate
the NMR spectrum and use the integration values to help identify as many of
the peaks as you can. Pay special attention to the pair of doublets at
1.0-1.3 ppm because these are the resonances of the methyl protons of the
amine. Literature values of these chemical shifts are 1.23 ppm for the R-(+)
amine salt and 1.12 ppm for the S-(−) amine salt.
Thus the relative integrations of these two doublets provide a measurement of
ee for your product. (Convert the relative
integrations to percents and then calculate ee by
simple subtraction.)
Polarimeter Method. Measure
the specific rotation, [α], of the product using a polarimeter and a
10-mL graduated cylinder
(see procedures below). Also, look up the literature value of [α] for pure (−)-α-phenylethylamine.
Use the two values of [α] to determine the optical purity of the
sample (Chapter 14.5)
·
Weigh
a 10-mL graduated cylinder that has a glass (rather than plastic) base. We
need the bottom of the cylinder to be transparent because we will be using it
as a polarimeter tube.
·
Add all of your amine product (after having taken a sample for
the NMR determination of ee) to the graduated
cylinder.
Weigh again to get the mass (m) of amine.
·
Measure
the volume (v) of liquid in the cylinder using the gradations on the
cylinder.
·
Measure
the height of the column of liquid in the cylinder. This is the polarimeter
tube length (l).
·
Place
the graduated cylinder in the polarimeter and measure the rotation to the
nearest degree. Do three trials and average the result.
·
Use
the standard polarimetry equation (Chapter 14.4) to
calculate [α].
Report
Please try to integrate the answers to the assigned questions into your
results and conclusions section for this report (rather than answering each
sequentially).
Assigned Questions:
|
1.
What
is the reaction that occurs between the amine and carbon dioxide? Do some literature research to find the answer and draw
the structure of the product.
|
|
2.
Discuss
the advantages and disadvantages of each method for determination of
enantiomeric excess.
|
|


