|
Chemistry 350 Laboratory - Fall Semester 2010 – Professor T. Nalli, Winona
State University
Expt
#6. Synthesis
of 1,2-Dibromo-1,2-diphenylethane
Relevant textbook readings – Mohrig, Chapter 9. Smith, Chapter 10.13-10.14
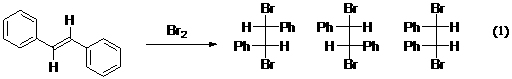
Overview – The stereoselectivity of alkene bromination
will be examined
in this experiment.
trans-1,2-Diphenylethene (common
name trans-stilbene)
will be brominated to form
1,2-dibromo-1,2-diphenylethane (eq 1). This product exists as three stereoisomers. You will determine which ones actually
form in the reaction. From this information you will determine if the
addition of Br2 is anti, syn, or nonstereoselective.
Procedures
Because Br2 is extremely hazardous, we
will generate it in situ in small
amounts by the reaction of potassium bromate (KBrO3)
with HBr (eq 2).
KBrO3
+ 6 HBr —› 3 Br2 +
KBr
+ 3 H2O (2)
Running the reaction.
·
Add
0.60 g of trans-stilbene
and 10 mL of hexanes to a 25-mL round bottom flask.
·
Clamp
the flask up to a ring stand well back into the fume hood so as to prevent
any accidental exposure to Br2 fumes that may escape the reaction
apparatus. Begin stirring the solution using a magnetic stir bar.
·
Add
0.40 g KBrO3 to the stirring mixture.
·
Attach
a reflux condenser and make sure water is running through it before going on
to the next step.
·
Quickly
add 1.6 mL 48% hydrobromic acid through the top of
the reflux condenser to the reaction flask.
·
Stir
the solution for 40 min at room temperature.
·
To
destroy any left-over bromine, add 1-pentene dropwise
until there is no trace of the red color of bromine remaining. (No more than 5 or 10 drops of should be necessary).
Work-up procedures.
·
Collect
the crystals by vacuum filtration on a pre-weighed Buchner funnel washing
with a small amount of ice-cold hexanes. (Hint: use cold hexanes to rinse any
crystals that remain in the flask into the funnel.)
·
Dry
the crystals somewhat by using the vacuum filtration apparatus to pull air
through them for a few minutes.
·
Weigh
the funnel with the crystals and calculate the yield of crude product.
·
Recrystallize (see Mohrig, Chapter 9) the
product from xylenes: Add the crystals to a 100-mL round
bottom flask along with 10 mL of xylenes. Add a few
boiling chips, attach a reflux condenser and heat the solution to boiling on
a sand bath. Add more xylenes slowly, maintaining
boiling, until all of the solid has dissolved. Cool
slowly to room temperature and then on ice and collect the crystals on a Buchner
funnel. Wash the crystals with 1-2 mL of cold hexanes.
·
Weigh
the final product and calculate the yield and percent yield.
Characterization
of Product
·
Obtain the melting point. Look up literature
values for the different stereoisomers of 1,2-dibromo-1,2-diphenylethane. Which stereoisomer do you
have?
·
Obtain the 1H NMR and 13C NMR spectra using CDCl3 as the solvent.
·
Obtain the IR spectrum using mineral oil (Nujol) as a carrier fluid. This type of IR sample is commonly referred to as a "Nujol mull".
Report
Please try to integrate the answers to the assigned questions into your
results and conclusions section for this report (rather than answering each
sequentially).
Assigned Questions:
|
1.
Which
stereoisomer was formed in this reaction? Explain this result by using the accepted mechanism for bromination of an alkene by Br2.
2.
What was the purpose of washing the crystals in each vacuum filtration step with cold hexanes? Why does the wash solvent have to be cold?
|
| |
|
