|
Chemistry 350 Laboratory - Fall Semester 2011 – Professor T. Nalli, Winona State University last revision 12/1/2011 Expt #5. Dehydrohalogenation of
2-Bromoheptane Relevant textbook readings – Mohrig,
Chapter 11, 13, 13. Klein, Chapter 8 Overview
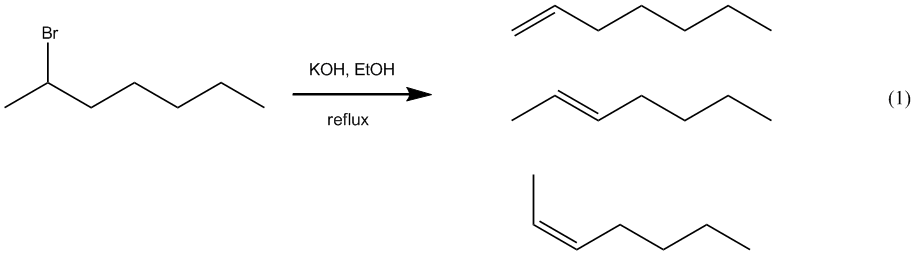
– In this experiment, we will react 2-bromoheptane with the strong base, potassium
hydroxide, to obtain an alkene product (eq 1). The
expected mechanism for this reaction is E2 and three different alkenes are
possible. The main goals of this experiment are to determine which alkene is actually
formed as the major product and to determine amounts of any minor products that are also formed.
Procedures Safety Wear gloves when measuring out the
reactants and throughout the extraction procedures. Ethanolic
KOH is very caustic and skin contact should be avoided. If you do get KOH on
your skin (you will not immediately feel a burning sensation but you can tell
it’s there by a slippery/soapy sensation on your skin) make sure to
immediately rinse with cold water for at least five minutes. Running the reaction Add 2.5 g KOH, 3.2 mL 2-bromoheptane, 5 mL of
95% ethanol, and a stir bar to a 25-mL round bottom flask (rbf). Stir or swirl until almost all of the KOH has
dissolved. Attach a condenser and proceed to reflux the solution for 60 min. Work-up Cool the solution to
near room temperature and then transfer it into a test tube containing 5 mL
of cold water. Cap the tube and carefully mix and shake the contents being
careful to vent frequently. Let the tube stand for 5 min. For the procedures in the following paragraph, make sure
to identify the organic and aqueous layers correctly! See section 11.2 –
“Practical Advice on Extractions” in Mohrig. You
can also test separated aqueous layers by adding a few drops of water to them
to make sure that the water dissolves in. If the presumed aqueous layer is
actually organic then the added water drops will not dissolve. Use a pipet to
transfer the organic layer to another test tube. Then wash the organic layer
with 5 mL H2O being careful to vent as needed. Allow the layers to
separate and pipet out the water layer into a waste beaker to be discarded
later. (As a general rule, never discard any material from a reaction until
the final purified product is obtained and verified). Wash the organic layer
with 5 mL H2O twice more, each time transferring the aqueous layer
into the waste beaker. Dry the organic layer
over sodium sulfate. Use a Pasteur pipet to remove the liquid from the drying
agent and transfer it into a dry, pre-weighed vial.
Weigh the vial with the product to
determine the yield.
Characterization
of Product
Record an IR and proton NMR spectrum of the product. Prepare a sample for GC-MS analysis by diluting 1-2 drops of your NMR sample in 5 mL dichloromethane in a clean labeled vial. One group will also be asked to obtain a C-13 NMR of their product. Report - A full report is required for this lab. Assigned Questions 1. What is the most likely side reaction in this experiment? 2. How would using a bulky base such as potassium tert-butoxide alter the results of this experiment? |