Expt
2. Acid/Base Extraction, Recrystallization,
Sublimation, and C-13 NMR.
Part
1 - Acid/Base Extraction
Reading Assignment - Mohrig Chapter 10, 11,
12
Background
In
this experiment you will learn how to use a separatory
funnel for the purpose of carrying out liquid/liquid
extraction, a useful separation method commonly
referred to as just "extraction". The process
of extraction involves intimately mixing a solution
with an immiscible extraction solvent so as to allow
compounds in the solution to partition between the two
liquid layers that form after mixing is discontinued.
Compounds that are more soluble in the extraction
solvent than in the original solvent will end up
predominantly in that layer, whereas compounds that
are less soluble will stay in the original solvent.
Water is almost always one of the solvents with the
other solvent being an organic compound such as
diethylether (CH3CH2OCH2CH3)
or dichloromethane (CH2Cl2), so
the two layers can be referred to simply as the
aqueous layer and the organic layer. Physical
separation of the layers then accomplishes the
separation of the compounds in the solution based on
their solubility characteristics.
Acid/base
extraction is a process that allows the separation of
organic acids, organic bases, and organic neutral
compounds (not an acid or base) from each other based
on the solubility differences of the organic acid (or
base) and its conjugate base (or conjugate acid).
Organic acids such as carboxylic acids (RCOOH),
phenols (PhOH), and thiols (RSH), all have an acidic
proton that can be deprotonated by aqueous base
(usually NaOH) to form a salt form of the acid, which
is much more soluble in aqueous solution than in
organic solvents, as illustrated by equation 1 for a
carboxylic acid. Hence, the carboxylic acid can be
extracted from an organic solvent by aqueous NaOH.
RCOOH + NaOH(aq)
→ RCOO-Na+(aq)
+ HOH (1)
The
original carboxylic acid can be retrieved from the
aqueous layer by simply neutralizing the base with
HCl(aq) and reforming the carboxylic acid (eq 2). The
relatively insoluble carboxylic acid often
precipitates at this point and can be collected by
vacuum filtration.
RCOO-Na+(aq) + HCl(aq) → RCOOH(s) + NaCl(aq) (2)
Conversely,
organic bases (e.g., amines, RNH2, R2NH,
or R3N) are protonated by aqueous acid
(usually HCl) to form salts that are much more soluble
in the aqueous layer (eq 3). Hence, amines can be
extracted from an organic solvent by aqueous HCl.
RNH2 + HCl(aq) →
RNH3+Cl-(aq)
(3)
The
original amine is retrieved by treating the aqueous
layer with aqueous base (NaOH) so as to deprotonate
the salt (eq 4), which often precipitates and can be
collected by vacuum filtration.
RNH3+Cl-(aq) + NaOH(aq) → RNH2(s) + HOH + NaCl(aq) (4)
Organic compounds that are neither acids or bases do not react with either NaOH or HCl and, therefore remain more soluble in the organic solvent and are not extracted.
Overview
You will use an acid/base extraction to separate a mixture of ibuprofen, caffeine, and the mosquito repellant, DEET. We will use the second week of the lab to purify the separated compounds using recrystallization or sublimation and then test their purities by mp determination.
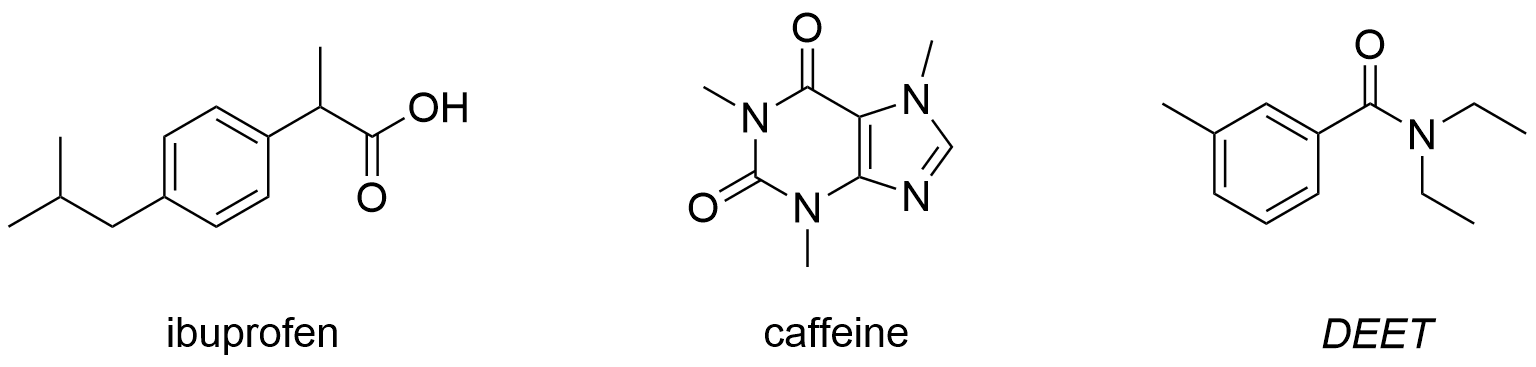
Pre-Lab
The
mixture given includes an organic acid, an organic
base, and a (non-acid non-base) neutral compound. Identify
which is which and write equations for the reactions
that will occur when the mixture is extracted with HCl
and then later with NaOH. Also,
make sure to include a literature pKa value for the
acid and pKb for the base in your table of reactants
and products. Your
planned procedures should explicitly state which
compounds are obtained in the respective steps 11, 13,
and 15.
Experimental Procedures
- Dissolve 1.5 g of the provided mixture in 20 mL dichloromethane (DCM) and transfer the contents to a separatory funnel.
- For steps 3-7 pay
special attention to the information in chap 11.2
of Mohrig and to the instructions provided by the
instructor on the proper use of the sep funnel.
Also see https://www.youtube.com/watch?v=2A98YEKzsMI
for a nice video tutorial. It is strongly
recommended that you label all containers used to
contain the various layers separated and solids
obtained.
- Add 15 mL 1M HCl. Cap the funnel and shake
gently at first with frequent venting. As it
becomes clear that excessive pressure is not
building up, end by shaking vigorously for 30 s or
more and then allow the layers to separate. Before
you go onto step 4 make sure you know which layer
is organic and which is aqueous.
- Into separate Erlenmeyer flasks, drain off the bottom layer through the stopcock and then pour out the top layer through the top of the sep funnel.
- Return the organic layer to the sep funnel and and extract it with another 15 mL of 1M HCl using the same procedures as before. Combine the aqueous layers from the first two extractions and return the organic layer to the sep funnel.
- Extract once more with 1M HCl, combining the obtained aqueous layer with that obtained previously.
- Return the organic layer to the separatory
funnel and extract it with 15 mL of 1M NaOH.
Separate the layers as before and return the organic
to the funnel.
- Extract the organic layer twice more each time with 15 mL of 1M NaOH combining the obtained aqueous layers with that obtained from the first bicarbonate extraction.
- Dry the organic layer over anhydrous Na2SO4. Remove the drying agent by simply decanting the liquid into a dry round bottom flask. See Chap 12 in Mohrig.
- Remove the DCM solvent on the rotary
evaporator (Mohrig, Fig 12.7), the instructor or TA
will assist with this.
- Use a Pasteur pipet to transfer as much of the obtained oily liquid as possible into a vial. Weigh and save for week 2.
- Carefully add 1M NaOH to the aqueous layer from the HCl extractions so as to neutralize the pH. You can use pH paper to make sure the solution is no longer acidic and/or you can use the formation of precipitate as a gauge. The organic base is not soluble in water so it precipitates as it is reformed from the salt by deprotonation. Thus, maximum precipitate formation indicates complete neutralization.
- Collect the formed solid by vacuum filtration on a Buchner funnel (Mohrig Chap 10.4). Weigh it and allow it to air dry until week 2.
- Use 1M HCl to neutralize the aqueous layer
from the NaOH extractions. Do the addition carefully
and slowly.
- Also collect this solid by vacuum filtration, weigh it and and allow to air dry until next week.
Assigned Questions
- The original mixture was prepared by using equal masses of the three compounds. Use this information to calculate the percent recovery of each compound. (The proper word here is "recovery" not "yield" because no net chemical reaction occurred. Instead we merely are recovering the unchanged components of the mixture.)
- Use the pKa's of the acids on both sides of your equations for the acid/base extraction reactions (see prelab assignment) to calculate the equilibrium constants for these reactions. Hint 1: the pKb of a base and it's conjugate acid are related by the equation, pKa + pKb = 14. Hint 2: You will need to look up values for the pKa of water and of HCl. Hint 3: You can use this equation to calculate pKeq from pKa's: pKeq = pKa(reactant) - pKa(product).
- Biochemists like to use the equation pKa
= pH + log([HA]/[A-]) to qualitatively
predict whether an acid (HA) is mostly
deprotonated (A-) or mostly in
its protonated form (HA). The idea is that if pH
< pKa (by at least one unit) then the log term
is greater than 1.0 meaning that [HA]/[A-]
is greater than 10. Thus, under these conditions
the acid is at least 90% in its protonated form.
Conversely, if pH > pKa then [HA]/[A-]
is less than 10 and
the acid is at least 90% deprotonated. In very
general terms, if the solution is more acidic than
the acid is (i.e., pH < pKa) then the acid is
forced to stay protonated, but if the solution is
less acidic than the acid (pH > pKa) then the
acid will be fully deprotonated. Question:
Calculate the pH of 1M NaOH(aq) and use it to
determine if the organic acid was fully
deprotonated in steps 7 and 8 of the extraction
procedure.
- What do your answers to questions 2 and 3 tell us about how much of the carboxylic acid and amine should theoretically have reacted when reacted with NaOH(aq) and HCl(aq) respectively?