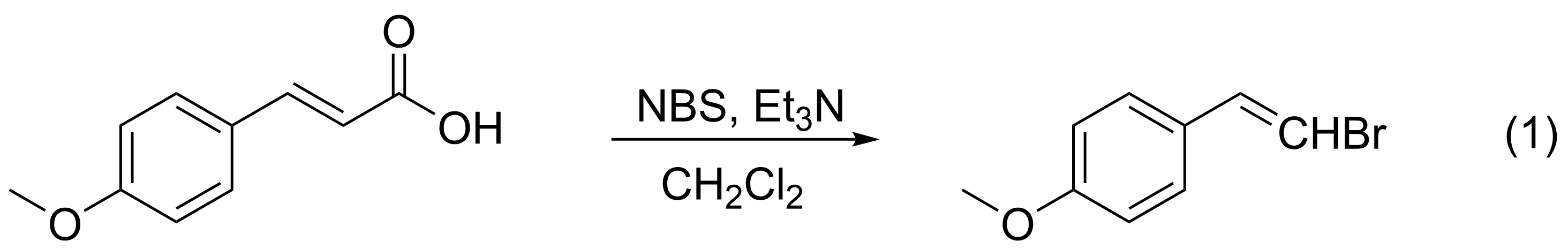|
Expt 6. Preparation of
1-(2-bromoethenyl)-4-methoxybenzene
revised 120418
Relevant textbook readings – Mohrig, Chapter
21.9. Klein, Chapter 9
Literature Reference - Evans, T. A. J.
Chem. Educ. 2006, 83, 1062.
Overview
In this lab 4-methoxycinnamic acid will be reacted
with N-bromosuccinimide (NBS) in the presence of
catalytic triethylamine. The cinnamic acid undergoes
a substitution reaction of Br for CO2H
under these conditions and thus gives
1-(2-bromoethenyl)-4-methoxybenzene (eq 1).
Triethylamine catalyzes the reaction by
deprotonating the carboxylic acid forming an
alkenylcarboxylate, which reacts quickly with NBS to
form a monobrominated neutral intermediate. (NBS
functions as a source of positive bromine (Br+)
in this reaction.) The intermediate then loses CO2
to form the final product, which can exist as both
an E and a Z isomer..
Procedure
Weigh
0.18 g 4-methoxycinnamic
acid and 0.21 g NBS into a 5 or 6-mL 14/10
reaction vial equipped with a magnetic spin vane.
Add
3 mL
CH2Cl2 followed by 10 μL of
triethylamine.
Stir
vigorously until the solids dissolve, approx
5–10 min.
Continue
stirring while monitoring reaction progress both
by TLC (CH2Cl2)
as well as by paying attention to evidence of CO2
evolution.
After the reaction is complete as
indicated by TLC, use a chromatography column dry
packed with silica gel (height of silica gel in
column = approx 10 cm) to remove succinimide,
NBS and triethylamine from the product solution.
First run hexane (15 mL) through the column so as to
wash off any impurities present in the silica gel.
Then add the reaction solution followed by 15 mL CH2Cl2.
Dry
the CH2Cl2
solution over Na2SO4
and remove the solvent on the rotary evaporator.
Determine
the mp and obtain a 1H NMR spectrum of
the product. One group will asked to be obtain a
C-13 NMR spectrum.
Questions
- Based on the observed mp as well
as the coupling constants observed for the alkene
doublets as compared to literature values, which
stereoisomer of 1-(2-bromoethenyl)-4-methoxybenzene
was formed?
- Propose a mechanism for the reaction that accounts
for the observed stereoselectivity.
- The proton NMR of the product shows clear evidence
for the presence of an impurity in the form of small
doublets at 5.4, 6.0, 6.9, and 7.4 ppm. Propose an
identity for this impurity and suggest a mechanism
for its formation.
|