CHEM 340 Organic Chemistry Survey, Prof. T Nalli, Fall 2003 Winona State University
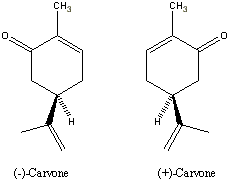
Experiment #5 - Comparison of Enantiomers: (+)-Carvone and (-)-Carvone
References:
McMurry, Chapter 6; Lehman, Operations 32 (p 197), 33 (p 200), 37b (p 257)

Overview - The physical and biological properties of the enantiomers, (+)- carvone and (-)-carvone, will be compared. Physical properties to be measured are the refractive index, the specific rotation, and the C-13 spectrum. The odors of the two carvones will also be assessed.
Safety Precautions - CDCl3 has harmful fumes. Avoid breathing it and dispense it in a fume hood.Procedures:
NMR procedure. You will be assigned one of the two carvones. See the handout for experiment #2 and your lab notebook for the procedures used for sample preparation. The instructor will assist in obtaining the C-13/DEPT spectrum of your compound. Dump out the tube into the halogenated waste solvents container and rinse it several times with acetone into the waste acetone container. Store it uncapped in your drawer.
Specific rotation procedure. The polarimeter you will use is different from the one described in Lehman. It does use a sodium lamp as the light source. With the sample tube inserted you should rotate the eyepiece to achieve maximum darkness in the center of the field of vision. Negative rotations on this polarimeter are calculated by subtracting the reading from 360 degrees. First measure the rotation of a blank sample (water). Do three independent measurements. If the average is significantly off from 0º then you are doing something wrong. Then measure the carvone samples the same way. Note the temperature in the lab in the vicinity of the polarimeter - we will assume the temperature of the samples is the same as the room. Use a ruler to carefully measure the height of each liquid in the polarimeter tube.
Refractive index procedure. The procedure is similar to the one given in Lehman on pp 199-200.
(1) Turn the refractometer on using the switch at front bottom right of the instrument.
(2) Make sure the mode selector is set to nD.
(3) Open the prism assembly cover. If the measuring prism surface is not clean then it should be cleaned with alcohol and then distilled water (see step 12). Any residue left on the prism will affect the accuracy of readings.
(4) Apply 4-5 drops of the sample solution to the measuring prism surface using a pipet. Important: the face of the prism must be completely covered with liquid. Close the prism cover and position the illumination arm so that the exposed face of the upper prism is fully illuminated.
(5) Turn the dispersion correction wheel (see diagram) so that the crosshair adjustment access hole is at the six o'clock position.
(6) Rotate the coarse adjustment control (large knob on right hand side) to position the shadow line at the bottom of the field of view.
(7) Rotate the eyepiece to bring the crosshair into focus (if it isn't already).
(8) Move the shadow line to the crosshair reticle with the coarse adjustment control.
(9) Rotate the dispersion correction wheel to eliminate any red or green color at the edge of the shadow line.
(10) Turn the adjustment control to center the shadow line to the crosshair. The shadow line must be perfectly centered to obtain an accurate reading (Figure 3a).
(11) Depress the READ button. The value of the test sample will be digitally indicated in the display window. Depressing the TEMP button will activate a temperature-sensing device located in the measuring prism. The display will digitally indicate the actual temperature of the measuring prism and sample. (Always record the temperature as well as the nD reading..)
(12) Open the prism assembly cover and clean the measuring prism surface with alcohol and then distilled water. Wipe with a clean soft cloth or lens tissue, but do not wipe the measuring prism surface while it is dry.
Odor assessment procedure.
Early in the lab (before your sense of smell becomes overwhelmed) you should try to carefully detect and describe the odor of each carvone. Compare the odor to other substances familiar to you but qualify the comparison if necessary. Do not stick your nose in the bottles and sniff! Not only is this dangerous, it will be counterproductive as your olfactory sense is keenest when detecting lower levels of compound.
Report:
Present all of the results except NMR results in one table. Make sure you convert the observed optical rotations to specific rotations before reporting. Use the equation given at the top of p 199 to correct the nD readings to the standard temperature of 20ºC. Include literature values for the refractive index and specific rotation and include the % error of each measurement in the table as well.
Make a separate table of NMR results that lists the data for both carvones, assigns each peak, and gives literature value. The table should make comparing the spectra of the two carvones easy.
Questions/Discussion Points
1. Discuss the accuracy and precision of the refractive index and optical rotation measurements. Discuss sources of error in each.
2. What did this experiment prove about the properties of enantiomers? Discuss at some length please.