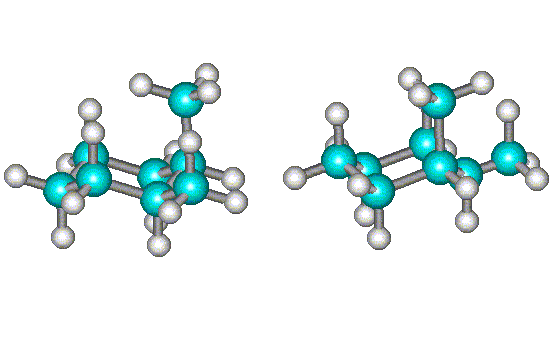
cis-1,2-Dimethylcyclohexane
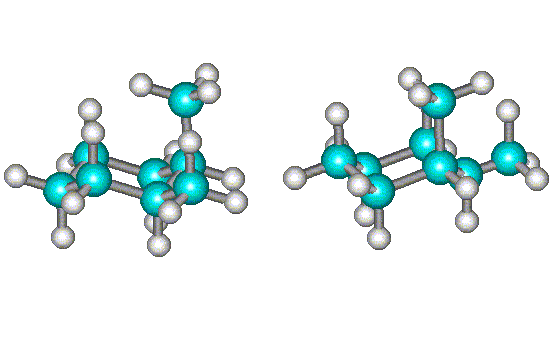
The two structures shown above are interconverted by a chair flip (conformations). Although they both have one Me group equatorial and one Me group axial, they are not superimposable and are mirror images (see below). Therefore, this compound exists as a pair of conformational enantiomers and is optically inactive (and usually considered achiral).
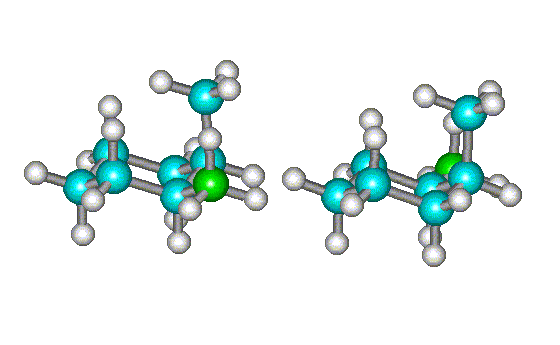 |
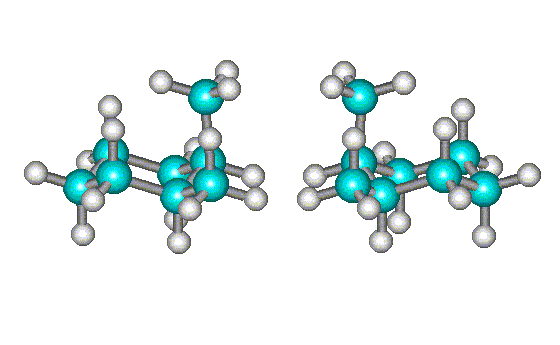 |
| Rotating the conformation at right shows that it is not superimposable on the first one - the equatorial methyl groups (shown in green) do not line up. | Further rotation of the conformation at right shows that it is the mirror image of the first one. Hence, they are enantiomers (but are interconverted by single bond rotations and are, therefore, conformational enantiomers). |