

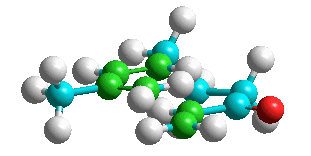

The reactant shown above (9-methyl-1,7,9-decatrien-3-one) possesses both a conjugated diene part and a dienophile part (carbons shown in green in the models). Furthermore, single bond rotations allow it to get into a conformation (like the one shown in the middle) that brings the diene and dienophile sections into proximity. Intramolecular Diels-Alder cycloaddition gives the bicyclic product shown (can you name it?) (issues of stereochemistry are neglected here). Click on the image of each model for an interactive version (requires the HyperChem WebViewer plug-in).