| The stereoisomers of 2,4-hexadiene react
as dienes in Diels-Alder reactions at very different
rates because of their varying abilities to exist in the
s-cis conformation.(For interactive models of each structure just click
on them - requires the HyperChem WebViewer plug-in) |
isomer
|
s-trans or transoid
conformation (double bond carbons shown in
green)
|
s-cis or cisoid
conformation (double bond carbons shown in
green)
|
Comments (energy
differences cited are based on molecular mechanics
calculations)
|
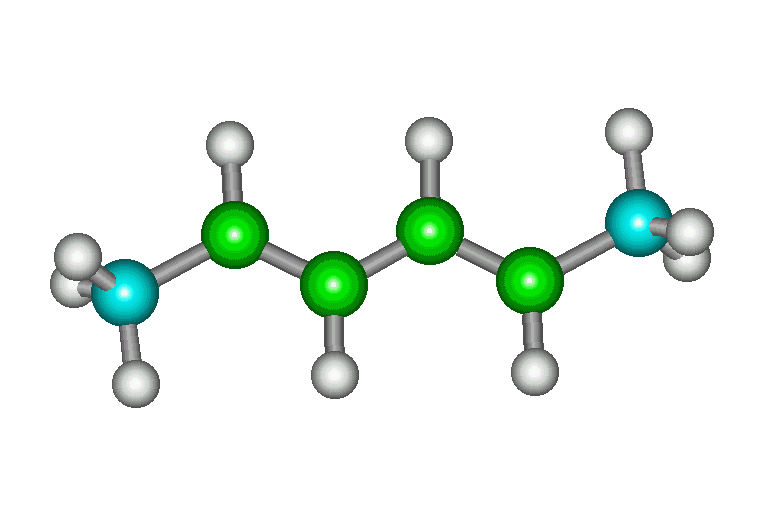
| trans, trans |
 |
 |
The s-cis conformation is only slightly higher in
energy than the s-trans (3 kcal/mol). Therefore, a fairly
high proportion of the molecules exist as s-cis and
Diels-Alder reaction is fast. |
| cis, trans |
 |
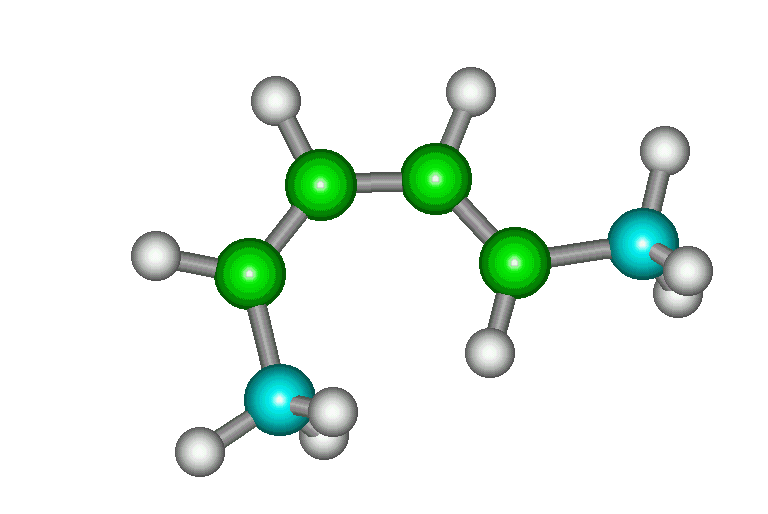 |
The s-cis conformation is moderately higher in energy
than the s-trans (6 kcal/mol). Therefore, a lower
proportion of the molecules exist as s-cis and
Diels-Alder reaction is slower than with
trans, trans. |
| cis, cis |
 |
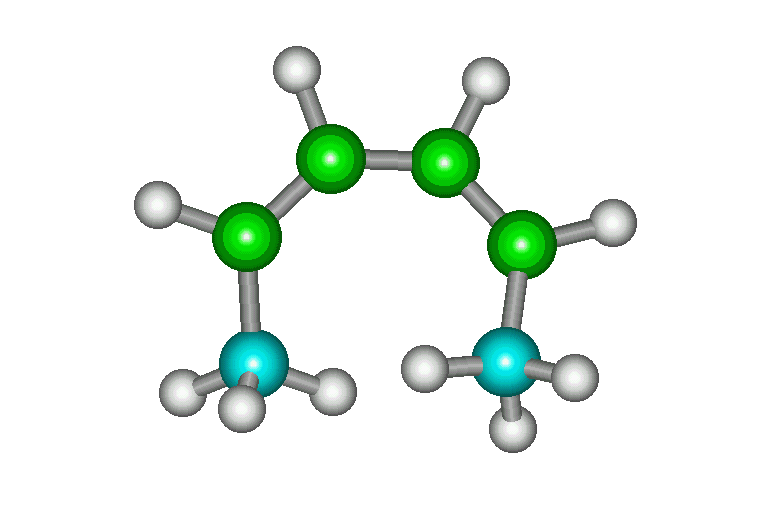 |
The s-cis conformation is much higher in energy than
the s-trans (11 kcal/mol) due to steric strain between
the methyls. Therefore, a very low proportion of the
molecules exist as s-cis and Diels-Alder reaction is very
slow. |





