Chemistry 351 Laboratory - Spring Semester 2006
Relevant textbook readings - Smith pp 561-569.
Prelab - Make sure you calculate the volumes to use of each of the reactants and include it in your table of reactants and products. Also make sure to include the theoretical yield of the product. As an additional assignment for this pre-lab, a prediction of the 1H NMR spectrum of the product is required. List the peaks expected along with their approximate chemical shifts, multiplicities and integrals.
Report - In lieu of a formal report for this experiment each group will simply turn in their product in a labeled baggie along with a completely labeled 1H NMR spectrum. (To completely label a spectrum, you have to draw the structure of the compound and identify all peaks in addition to all necessary identifying information.) In addition, the in-lab notes should conclude with a page that clearly reports the yield, %yield, mp and lit mp of the product.
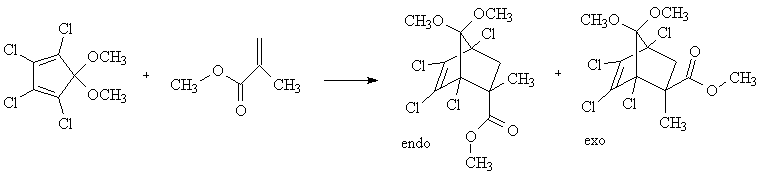
Overview - You will carry out the Diels-Alder reaction of 1,2,3,4-tetrachloro-5,5-dimethoxy-1,3-cyclopentadiene with methyl methacrylate. The equation is shown below. The reaction will be carried out as a solvent-free, microwave-enhanced process. The product (7,7-dimethoxy-2-carbomethoxy-2-methyl-1,4,5,6-tetrachlorobicyclo[2.2.1]hept-5-ene) will be characterized by 1H and 13C NMR and mp (lit mp 100-102).

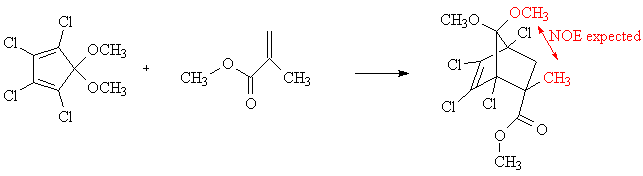
We can predict that the reaction will be stereoselective with the major product in the endo configuration. This prediction comes from the endo rule, which is explained by bonding interactions in the transition state between pi bond substituents on the dienophile and the p orbitals at carbons 2 and 3 of the diene. We will test this prediction by obtaining a pfg-NOE spectrum of the product. This experiment will demonstrate the proximity of the methyl protons shown in the equation below in red.
The nuclear Overhauser effect (NOE) is the name for a through-space effect, in which stimulating the resonance of one nucleus affects the spin-state populations of a nearby nucleus. The population of the higher-energy spin state of the nearby nucleus is increased, allowing it to release more energy than otherwise when it relaxes back down. In essence, stimulating the resonance of one proton by irradiating it with its resonance frequency will increase the intensity of a nearby proton's peak in the NMR spectrum.
Here is a link to a molecular model of the endo product. You should either download and install the HyperChem Webviewer plug-in so that you can view this model or build a model for yourself using ChemOffice.
Procedure
1. Add 1.0 mmol of the diene and 1.0 mmol of the dienophile to a 4-mL conical vial. Measure out the compounds by volume using a pipet but also measure the mass of each reactant used by weighing the vial before and after each addition.
2. Cap the vial with a tight-fitting rubber septum and label it with your group name(s).
3. Place in the microwave oven along with the other lab groups' vials.
4. When all reaction mixtures are ready to go in the microwave, we will microwave them on high for 10-15 minutes.
5. After cooling, recrystallize the product using hexane as the solvent.
6. Obtain a mp and 1H NMR spectrum. If time permits a 13C NMR may also be obtained.
7. The instructor will obtain a pfgNOE spectrum on one group's product and present the results to the class in lecture.
Literature Reference
Thompson. H. W; Wong, J. K.: Lalancett, R. A.: Boyko, J. A.; Robertiello, A. M. J. Org. Chem. 1985, 50, 2115-2121.