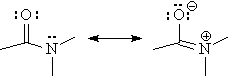
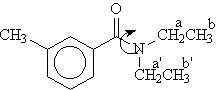
Expt #9 - Synthesis of DEET and Variable Temperature NMR Study of Amide Bond Rotation
Part 1. Synthesis
We will carry out the procedure essentially as described in Pavia, pp 385-387. Use a sand bath rather than an aluminum block for heating. No gas trap (with wetted glass wool) is required. We do not have long needles for the syringes so just try to make sure the drops from the syringe fall directly onto the surface of the liquid in the reaction flask (rather than dribbling down the sides of the glassware.)
Part 2. Molecular modeling and NMR study of amide bond rotation in DEET
Background
The energy barrier to rotation about most carbon-carbon single bonds is usually on the order of 3-5 kcal/mol. This low barrier means that these bonds freely rotate. For instance, the C-C bond of ethane rotates approximately than 1011 times per second at room temperature.
However, rotation about the the C-N bond of an amide is much less "free". This is due to the amide resonance which makes the C-N bond have appreciable double bond character. In other words, rotating the C-N bond destroys the pi overlap between the carbonyl carbon and the nitrogen resulting in a much higher energy barrier (Ea) for bond rotation.
The rate of a chemical reaction is related to the activation energy (Ea) according to the Arrhenius equation.
k = A × e-Ea/RT
A is the Arrhenius factor, which is approximately 2 x 1013.for the reactions being considered here. R is the gas constant = 0.001987 kcal mol-1 K-1 and T is the temperature in Kelvins.
Nuclear magnetic resonance occurs on a fairly slow time scale. Therefore, fast reactions are not discernable by NMR and the observed spectrum is the average spectrum of the interconverting molecules. In general, an averaged spectrum is observed when the rate of the process is 2p times the frequency difference (2p × Dn) between the peaks being observed. For example, consider a hypothetical molecule with two nonequivalent methyl groups with 1H NMR chemical shifts of 3.0 and 3.5 ppm on a 300 MHz instrument. The frequency difference between the two peaks is 150 Hz (0.5 ppm x 300 Hz/ppm). If a bond rotation (or any other chemical reaction) interconverted these methyl groups significantly faster than 900 times per second (900 s-1) (2p × 150 Hz) (Hz = s-1) then these six protons would show up as a single peak at 3.25 ppm. In contrast, if the methyl groups interconvert much slower than 900 times per second then they will show up as two distinct peaks at 3.0 and 3.5 ppm. When the interconversion rate is about equal to 2p × Dn the six methyl protons will appear as a single broad peak at the average chemical shift (3.25 ppm).
Increasing the temperature increases the rate of a chemical reaction according to the Arrhenius equation (given above). Therefore, in certain cases it is possible to time a chemical process by finding the temperature at which averaged spectra for the interconverting groups are obtained. At lower temperatures the process is slower and separate peaks are observed. At higher temperatures, one sharp peak at the average chemical shift value is obtained. This is what we will do for the C-N bond rotation in DEET.
We will find the temperature at which the two ethyl groups of DEET start to form one peak. Remember at this temperature, the rate of bond rotation (k) is approximately 2p × Dn.
Hence, we will know k, A, R and T so we can calculate Ea using the rearranged Arhennius equation:
Ea = ln (A/k) x RT
The value of Ea obtained from the NMR experiment will be compared to the predicted value from semi-empirical molecular orbital calculations using HyperChem.
Procedures
NMR. One student group's product will be used for the NMR study. The instructor will demonstrate the operation of the NMR in variable temperature (VT) mode. The spectra obtained will be posted in the class storage folder on the network as usual. You will process and print out spectra using MestRe-C and estimate the temperatures at which the CH3 triplets and CH2 quartets start to coalesce.
Molecular Modeling. In HyperChem build a model of diethylaminobenzamide (DEAB). (This is DEET omitting the meta-methyl group, which unnecessarily complicates the calculations.) Go to help and run the tutorial under "exploring conformations" on how to do 'torsional potential" calculations. Use this procedure to run a torsional potential calculation for the C-N bond in DEAB. You will need to select four atoms that define this bond torsion, the O, C of the C=O, N, and a C of one of the CH2 groups should do it. Run the calculation starting from a torsional angle of 0 degrees and ending at 180 degrees. Print out the graph that results.
Report Guidelines. As usual, start with results tables that summarize all of the results. Included should be yield, NMR, IR, GC-MS (printouts will be provided), and molecular modeling/VT NMR results. The NMR table should assign all of the peaks in the lowest temperature spectrum and give integrals and multiplicities. Also give literature values in the tables. The molecular modeling/VT NMR table should give the temperature of coalescence for each set of peaks that did coalesce and the corresponding calculated values for k and Ea. Compare the values of Ea to the value obtained by molecular mechanics calculations on HyperChem.
Discuss each table briefly immediately. State the most important results being presented by the table.
Show the yield calculations and the Ea calculations.
The conclusions section should present the mechanism of the reactions, discuss the purity and yield of the product, discuss how the various spectra prove the structure of the product, and discuss the molecular modeling and VT NMR. The following are some questions to help guide your writing of the conclusions section:
(1) How pure is the product? Are any impurities detectable in any of the spectra? If so, hypothesize as to the possible identities of the impurities.
(2) Was a 100% yield obtained? Was a 100% yield expected? If less than 100%, identify some places in the procedure where product was lost an discuss how the loss could have been minimized.
(3) How well does the Ea calculated using Hyperchem agree with the experimental values from the VT NMR experiment? Try to find a literature value for the Ea for amide bond rotation. How well do the results of this experiment agree with the literature value?
All of the spectra should be attached to the report, including printouts of the NMR spectra at all of the temperatures. (The NMR data is in the shared folder of our network class storage.)