Dr. Nalli's Chemistry 351 Laboratory - Spring Semester 2009
Relevant textbook readings - Smith - chapter 16.12-16.14. Lab Manual - Technique 11, pp 189-210.
Prelab - Make sure you calculate the volume of phellandrene and the mass of maleic anhydride to use and include both in your table of reactants and products. Also make sure to include the theoretical yield of the product. As an additional assignment for this pre-lab, a prediction of the 13C NMR spectrum of the product is required. List the peaks expected along with their approximate chemical shifts.
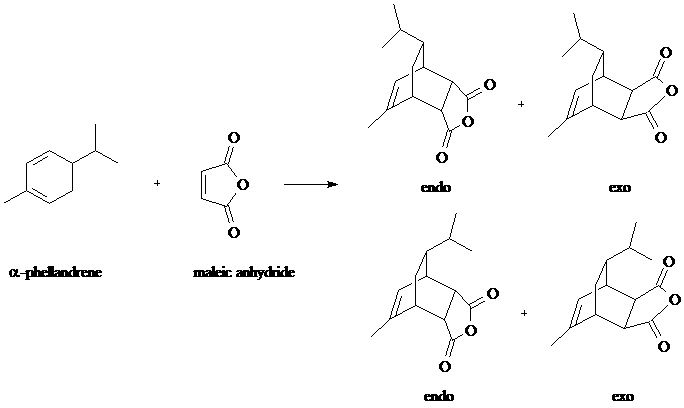
Overview - You will carry out the Diels-Alder reaction of the terpene natural product, alpha-Phellandrene, with Maleic Anhydride. The resulting product could conceivably be any of four possible diastereomers as shown in the equation below.

We can predict that the reaction will be stereoselective, forming predominantly the stereoisomer at top left. We dismiss the bottom two structures based on prohibitive steric hindrance from the isopropyl group and, based on the endo rule, we expect an endo rather than exo product. The endo rule is explained by bonding interactions in the transition state between pi bond substituents on the dienophile (in this case the C=O groups) and the p orbitals at carbons 2 and 3 of the diene.
We will run the reaction and obtain a crystalline product that will be thoroughly characterized by 1H, 13C, DEPT, and HMQC NMR. An IR spectrum and mp will also be obtained (lit mp 126-127). We will also attempt to demonstrate that the product is indeed the predicted stereoisomer by obtaining a pfg-NOE spectrum of the product. This experiment will demonstrate the proximity of the protons shown in the equation below in red.
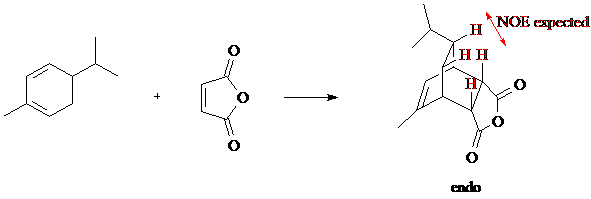
The nuclear Overhauser effect (NOE) is the name for a through-space effect, in which stimulating the resonance of one nucleus affects the spin-state populations of a nearby nucleus. The population of the higher-energy spin state of the nearby nucleus is increased, allowing it to release more energy than otherwise when it relaxes back down. In essence, stimulating the resonance of one proton by irradiating it with its resonance frequency will increase the intensity of a nearby proton's peak in the NMR spectrum.
Procedure
Reaction:
Modeling: