Chemistry 351 Laboratory - Spring Semester 2012
Expt #5. Grignard Synthesis of a Fluorinated Triphenylmethanol.
Relevant textbook readings - Mohrig Chapter 7.2-7.3. Klein Chapter 13.6.
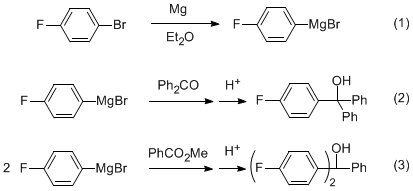
Overview - You will prepare a Grignard reagent from 1-bromo-4-fluorobenzene (eq 1) and then react it with either benzophenone to form 4-fluorotriphenylmethanol (eq 2) or with methyl benzoate to form 4,4'-difluorotriphenylmethanol (eq 3). The product will be analyzed by IR, 1H NMR and 13C NMR.
Procedures
Preparation of the Grignard Reagent (eq 1)
All of the glassware needs to be very dry for this reaction to work, so the instructor will supply oven-dried kits to each lab group at the start of the lab. Each kit should contain: a 25-mL round bottom flask, two large screw top vials, a 50-mL Erlenmeyer, a Claisen adapter, a drying tube, and a Pasteur pipet. Roughly calibrate the pipet by making marks with a wax pencil or Sharpie as shown on page 45 in Mohrig.
Assemble a reaction apparatus suitable for the slow addition of a reagent under anhydrous conditions (Mohrig, Chap 7.5a). The basis for this apparatus is shown in fig 7.5a. However, a drying tube filled with CaCl2 needs to be placed at the top of the reflux condenser (similar to the setup shown in fig 7.2b). Also, you will need to use a 25-mL round bottom flask in place of the conical vial shown in fig 7.3. (Also note that the syringe does not get inserted until you are actually ready to begin adding a reagent using it.)
Temporarily disassemble the reaction apparatus so that you can add 0.15 g of magnesium and a magnetic stirbar to the round bottom flask. Reassemble the apparatus.
Obtain 20 mL anhydrous ethyl ether using one of your dry vials. This is your stock of ether for the first part of the reaction. Keep the vial capped when not actively removing ether from it.
Weigh your other dry vial with cap. Add 0.75 mL 1-bromo-4-fluorobenzene (BFB) to it and reweigh it to get the actual mass used. Add 4.0 mL ether (from your stock vial) and swirl the contents to get the BFB to dissolve.
Add 1.0 mL ether (by syringe) to the reaction flask and begin stirring.
Add 0.8 mL of the prepared BFB solution. Stir with slight warming (cupping the flask with your hand works well or you can use a warm water bath (40-45 °C) but take care to not get water into the flask!).
Watch carefully for signs that the Grignard reaction has commenced. These include bubbles coming off the surface of the metal, gray/brown cloudiness to the solution, and signs of corrosion on the surface of the metal. If the reaction has not started on its own within 10 min of warming, then you will need to "jump start" it.
To jump start the reaction, discontinue warming and disassemble the rb flask from the rest of the apparatus. Working quickly, carefully use a dry spatula or glass rod to crush or break in half some of the Mg pieces. Doing so exposes non-oxidized, reactive surfaces on the metal which then allow the reaction to begin.
Once the reaction has started begin adding the rest of the BFB solution over a period of about 15 minutes. Maintain a rate of addition such that the solution refluxes on its own without external heating. If the addition is done too rapidly then the reaction may threaten to become too vigorous, in which case you should cool the flask briefly on a cold water bath (just enough to slow down the reaction take care not to stop it entirely). If the addition is too slowly, the reaction will start to subside as it runs out of starting material. If this happens to the point of it appearing that the reaction may stop all together then it is important that you add more BFB solution and/or apply gentle warming to get it going again.
After all of the BFB solution has been added, add 2.0 mL ether to the vial it was in, rinsing to dissolve any remaining BFB and then add this rinse solution ot the reaction flask. The reaction will naturally start to die out once all of the BFB has been added. Once it starts to do this, but before it stops entirely, apply heat from a warm water bath so as to keep it refluxing. Reflux on the warm water bath for 15-20 min and then allow to cool to room temperature.
Reaction of the Grignard Reagent with Benzophenone (eq 2)
Make a solution of benzophenone in 2 mL anhydrous ether in the same vial you used for the BFB. Use 1.0 equiv of benzophenone (the same amount of moles as of BFB used). Add the benzophenone solution to the reaction flask in two portions. (There is no need to do these additions slowly.) Maintain stirring for 20 min and then go on to the work-up procedures.
Reaction of the Grignard Reagent with Methyl Benzoate (eq 3)
Make a solution of methyl benzoate in 2 mL anhydrous ether in the same vial you used for the BFB. Use 0.5 equiv of methyl benzoate (i.e., half the amount of moles of BFB used). Add the methyl benzoate solution to the reaction flask in two portions. (There is no need to do these additions slowly.) Maintain stirring for 20 min and then go on to the work-up procedures.
Work-up Procedures.
(Note: for the remaining procedures the ether does not need to be anhydrous.)
The reaction mixture must first be neutralized with acid. Cautiously add 6.0 mL of 6M HCl. This forms the alcohol from the alkoxide and also breaks down remaining magnesium. Once all solids are dissolved, transfer the entire contents of the flask to a separatory funnel. Add 5 mL ether to the separatory funnel so that you have a good size organic layer to work with.
Drain the lower aqueous layer into a flask or beaker and then transfer the organic layer through the top of the sep funnel into a dry Erlenmeyer flask.
Extract the aqueous layer with an additional 5 mL ether. Again drain off the aqueous and then transfer the organic layer into the dry Erlenmeyer flask containing the first ether layer. Dry the combined ether extracts over Na2SO4.
Decant the dried solution into a small beaker and remove the solvent by warming under a stream of nitrogen. The crude product is an oily red semi-solid. It contains numerous non-polar impurities including unreacted starting materials and biphenyl side products. These can be mostly removed by trituration with petroleum ether.
Trituration with Petroleum Ether. Add 2 mL petroleum ether to the crude product and use a glass rod or spatula to stir and manually mash the solid under the petroleum ether. (This type of purification procedure in which an impure solid is washed with a solvent in which it is insoluble is referred to as "trituration".) If a solid forms as a result then use vacuum filtration to collect the solid. If the product is still an oily material then use a pipet to draw off most of the pet ether and then add fresh pet ether and repeat the trituration. Continue in this manner until you have a filterable solid. Wash the filtered solid with a very small amount of ice-cold pet ether. If the solid product is dark orange to red in color then you should repeat the trituration/filtration procedure as many times as is necessary to obtain a lightly colored, powdery solid crude product.
Recrystallization. Recrystallize the crude solid from petroleum ether.
Characterization. Obtain the mp as well as 1H, 13C NMR, and IR spectra of the final product. .
Report
Feel free to integrate the answers to the assigned questions into your results and conclusions section for this report (rather than answering each sequentially).
Assigned Questons:
1. Fluorobenzene, 4,4'-difluorobiphenyl, and 4-fluorobenzoic acid are possible as side products in this reaction. How does each form (show equations)? How is each removed? Do the obtained spectra show any evidence for the presence of any of these in the final products?
2. Do the spectra show any evidence of unreacted starting materials or solvents as impurities? Elaborate.
3. Calculate the C-F coupling constants (J values) observed in the 13C NMR? How do they compare with literature values for C-F coupling constants? (Use published data for fluorobenzene to obtain the literature values.)