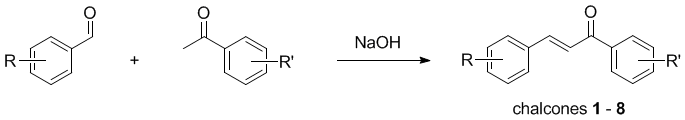Expt
#6. Solvent-Free Preparation of a
Fluorine-Substituted Chalcone
|
| Compound |
R |
R' |
| Chalcone |
H |
H |
| 1 |
4-F |
H |
| 2 |
4-F |
3,4
-O-CH2-O- |
| 3 |
4-F |
4-NO2 |
| 4 |
4-F |
4-Br |
| 5 |
3-NO2 | 4-F |
| 6 |
3,4 -O-CH2-O- | 4-F |
| 7 |
4-Me |
4-F |
| 8 |
4-NO2 | 4-F |
Procedures
We will use the procedure from reference 2 and copied below:
"In a typical reaction, 5 mmol of the benzaldehyde and 5 mmol of the acetophenone were added to a 3-inch porcelain mortar, 200 mg of solid NaOH (about a pellet) was added and the mixture ground with a pestle for about 5-10 minutes. Grinding was continued until the mixture solidified and the solid broke up in small particles. Distilled water (10 mL) was added and the product mixed well with the water, with the pestle and a spatula used to dislodge the solid from the mortar's wall. The suspension was vacuum filtered using a Buchner funnel. Mortar and pestle were rinsed with 5 mL of water and the rinses collected on the same filter. The product was washed on the filter with an additional 5-mL aliquot of water and it was allowed to air-dry on the filter."
Recrystallization. Carry out small-scale tests (Mohrig, pp 188-189) to determine the best recrystallization solvent from among 95% ethanol, toluene-ethanol, ethanol-water, or methanol and then recrystallize the remainder of the product from this solvent.
Characterization. Obtain the mp as well as 1H NMR, 13C NMR, and IR spectra of the final product.