Expt #2. Diels-Alder Reaction of α-Phellandrene with Maleic Anhydride
Relevant textbook readings -
Klein - chapter 17.7. Mohrig - chapter
15.
Literature Reference - Stockmann, H. J. Org. Chem. 1961 26, 2025-2029
Prelab - Make sure you calculate the volume of phellandrene and the mass of maleic anhydride to use and include both in your table of reactants and products. Also make sure to include the theoretical yield of the product.
Overview - You will carry out the Diels-Alder reaction of the terpene natural product, (R)-(–)-α-Phellandrene, with Maleic Anhydride. The resulting product could conceivably be any of four possible diastereomers, however, the configuration of C-5 of the phellandrene must be retained as shown in equation 1.
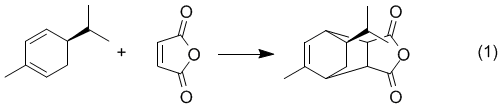
We will run the reaction and obtain a crystalline product that will be thoroughly characterized by 1H, 13C, DEPT, and HMQC NMR. An IR spectrum and mp will also be obtained (lit mp 126-127).
Procedure
Reaction:
- In a 25-mL round-bottom flask put 0.010 mole of maleic anhydride and 0.010 mole of α-phellandrene.
- Add 3.5 mL of ethyl acetate, attach a reflux condenser, and heat at reflux for 30 min.
- Allow to cool slowly to room temperature then cool on an ice bath to maximize crystal formation.
- Collect the crude product by vacuum filtration.
- Save a small sample of the crude product for a mp
determination.
Purification:
- Recrystallize the product from ethyl acetate. (Review chapter 15 in Mohrig).
- Get the crystals as dry as possible by sucking air through on the Buchner funnel for approx 15 minutes or by air drying for one week.
Analysis:
- Obtain the IR spectrum using the cast-film method of sample preparation. (see pp 286-287 in Mohrig).
- Obtain the mp of both the crude and recrystallized
product.
- Prepare a sample for NMR spectroscopy using acetone-d6 as solvent and obtain a proton NMR. The group with the highest quality proton NMR (as judged by the instructor) will also obtain the following spectra: 13C NMR, DEPT-135, HMQC, HMBC, or COSY. The instructor will assist and the other groups will report on their own proton NMR in conjunction with the additional spectra from the chosen group.