Expt #5. Part 2. Preparation of DEET from m-Toluic AcidRelevant textbook readings - Mohrig Chapter 7.2-7.3. Klein Chapter 21.6-21.8, 21.12. Literature References (1) Reaction procedures adapted from http://chemconnections.org/organic/chem227/deet-procedure-09.html (accessed Jan 21, 2013). (2) (a) Jensen, B. L.; Fort, R. C. Jr. J. Chem. Educ. 2001, 78, 538-540. (b) Gryff-Keller, A.; Szczeciński, P. Org. Mag. Res. 1978, 11, 258–261. Overview - The 3-methylbenzoic acid
prepared in part 1 of expt
5 will be chlorinated using thionyl
chloride and the product formed (m-toluoyl chloride) will be
reacted with diethylamine
hydrochloride and sodium hydroxide to form the
common insect repellant, N,N-diethyltoluamide
(DEET). The product will be characterized by proton
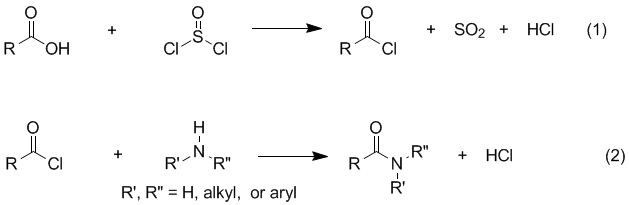
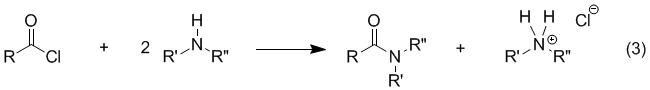
NMR and IR. Background Amides are easily prepared by treating a carboxylic acid with thionyl chloride to form an acyl chloride (eq 1), which can then be reacted with an amine to form the desired amide (eq 2). The reaction of the acyl chloride with the amine (eq 2) is usually very rapid and exothermic so that, in many cases, the rate must be controlled by cooling or other means. In the absence
of an added base, it is necessary to use 2.0 equiv of amine for eq 2 because the reaction
produces HCl, which
reacts rapidly with the amine to form an ammonium
salt (eq 3). Although
the reaction is often carried out in this manner
(using excess amine) it can be wasteful
(especially if the amine is valuable) and the
ammonium salt formed presents a product
purification problem depending on how difficult it
is to separate from the product. These
disadvantages can be avoided if a base such as
pyridine or sodium hydroxide is included as an
additional reactant.
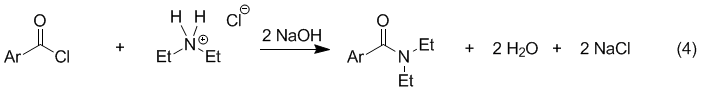
The Schotten-Baumann reaction is the variation of this reaction that uses aqueous sodium hydroxide as the base (eq 4). Water-soluble acyl chlorides are unsuitable reactants for this procedure because they will hydrolyze rapidly in water to form carboxylic acids (thus undoing eq 1). However, most aromatic acyl chlorides are practically insoluble in water and, therefore, hydrolyze much more slowly. The use of NaOH also makes it possible to use an amine hydrochloride rather than the free amine (e.g. Et2NH2+ Cl− instead of Et2NH) because the salt reacts with base to form the amine in situ. Many amines are corrosive and malodorous so the use of an amine salt is often advantageous.
A disadvantage of the Schotten-Baumann reaction is that very efficient mixing is necessary to provide adequate contact between the water-insoluble acyl chloride and the amine. To overcome this we will use a detergent known as sodium lauryl sulfate. Detergent action helps disperse the acyl chloride into smaller particle sizes that increase the reaction rate. Procedures
|