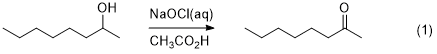Expt #1. Oxidation of 2-Octanol
Relevant textbook reading - Klein, Chapter
13.10, Mohrig, Technique 11.1-11.4, 16.3-16.4, 13
(especially 13.4).
Literature References - (a) Stevens,
R.V.,Chapman, K. T.,and Weller. H. N., J. Org.
Chem. 1980, 45,
2030-2032 (b) Mohrig,
J. R.; Nienhuis, D. M; Linck, C. F.; Van Zoeren, C.;
Fox, B. G.; Mahaffy, P. G. J. Chem. Educ.
1985, 62, 519-521
Overview – 2-Octanol will be oxidized using
hypochlorous acid (HOCl) (eq 1). The HOCl is generated in
situ from the reaction of acetic acid with aqueous
sodium hypochlorite (household bleach). The product will
be purified by vacuum distillation and characterized by
IR, 1H NMR, and refractive index
determination.
Background - The method we are using
for alcohol oxidation is regarded as a relatively
"Green" method because it does not employ heavy metal
based oxidizing agents (e.g., Cr or Mn) nor does it
generate toxic side products other than HCl, which is
easily neutralized with NaOH. It is often referred to as
the Chapman-Stevens oxidation in honor of the scientists
who first reported it.
The
mechanism has been proposed to start with a
proton transfer to the HOCl oxidant. The resulting H2OCl+
is attacked nucleophilically by the alcohol to generate
(after proton transfer) an ROCl intermediate, which then
undergoes an E2-like reaction to form the ketone.
In order for the oxidation to reach completion excess
HOCl must be used. However, the concentration of
hypochlorite in household bleach, often stated as 6.0%,
varies significantly. Therefore, the presence of excess
HOCl cannot be assured by simply using a predetermined
volume calculated based on the bleach concentration.
Instead, we will test for the presence of HOCl using
KI/starch test paper. This paper is impregnated with
iodide ions and starch and will turn black if treated
with an oxidizing agent (e.g. HOCl) which oxidizes the
iodide ions to iodine molecules (I2), which
in turn form a blue-black complex with the starch (eq
2).
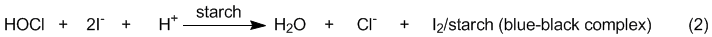
Pre-Lab - As always, your plan for this
experiment should provide a balanced equation for the
reaction being carried out (i.e., a balanced version of
eq 1) as well as molar amounts of all reactants and
theoretical mass yield of product.
Procedures
Running the reaction.
-
Place 0.025 mol of
2-octanol and a magnetic stir bar in an Erlenmeyer
flask.
- Carefully add 1.5 mL of glacial acetic acid to the
alcohol with stirring.
- Suspend a separatory funnel over top of the flask
using a metal ring.
- Add 35 mL of bleach (6.0% NaOCl) to the separatory
funnel. (Adjust the volume if the stated
concentration of the bleach is not 6.0%)
- Use the separatory funnel to add the bleach drop
by drop to the alcohol/acetic acid mixture over a
period of about 15 min. Monitor the temperature of
the solution during the addition and apply an ice
bath and/or slow the addition rate as necessary to
keep it in the range of 25-50 °C.
- When the addition of the bleach is complete, use a
KI/starch test strip to test the solution for excess
hypochlorous acid. If the test is negative then add
additional bleach. (See background above.)
- Stir the reaction mixture for an additional 30
min on a warm water bath at approx 50 °C, testing
with KI/starch paper every 5 min as above and adding
more bleach if necessary.
Work-up procedures.
- Destroy excess HOCl by adding 0.5 mL saturated
sodium bisulfite (NaHSO3). Test the
reaction for any remaining hypochlorous acid and, if
necessary, add additional sodium bisulfite.
- Add 5 drops of thymol blue (a pH indicator).
- Add enough 6M NaOH to turn the solution a light
blue (indicating a basic pH). (The approximate
volume of NaOH solution necessary for this step can
be calculated by simply equating the moles of NaOH
necessary to the moles of acetic acid started with.)
- Add 4.0 g solid NaCl and stir until it all
dissolves. (See the discussion of "salting out" in
Mohrig, chapter 11.)
- Decant the liquid into a separatory funnel and
extract with 10 mL diethyl ether (EtOEt) taking care
to vent frequently. (Review extraction procedures in
Mohrig, 11.1-11.4.)
- Transfer the organic layer to a labeled, dry
Erlenmeyer flask.
- Extract the aqueous layer two more times with 10
mL EtOEt each time combining the organic layer with
the original organic layer.
- Dry the organic extracts with Na2SO4
and decant the solution into a 50-mL round bottom
flask.
- Remove most of the ether solvent using the rotary
evaporator (Mohrig pp 139-140).
- Transfer the residue into a 5 or 10-mL round
bottom flask and carry out vacuum distillation of
the product. (see Technique 13.7, Fig 13.28 in
Mohrig.)
- Allow the distillation to proceed until the bp of
the distillate drops significantly.
Characterization
of Product
- Obtain the 1H NMR spectrum using CDCl3
as the solvent.
- Obtain an IR spectrum.
- Measure the refractive index of the product.
(Mohrig technique 16.3-16.4)
Report
- Report the spectroscopic results in tables as
usual (and as specified in the report guidelines).
For this report you will also need to report the
coupling constant for each NMR multiplet.
- Remember to also report the yield, theoretical
yield, and percent yield as well as the refractive
index and observed bp of the product. Use the
equation given in Mohrig to correct the measured
refractive index so it can be directly compared to
the literature value given at 20 °C.
Assigned
Questions
1. Write an equation for how the first step of the
work up procedures works to destroy left over HOCl.
Also propose a mechanism for this reaction.
2. What was the purpose of adding NaCl(s) before the
extraction with diethyl ether was carried out?
|