Expt 4. Green Wittig Synthesis; Introduction to APCI-MS
Relevant textbook readings - Mohrig, Chapter 17, Klein, pp 965-967.
Other References - (1) Morsch,
L. A.; Deak, L.; Tiburzi, D.; Schuster, H.; Meyer, B. J.
Chem. Ed. 2014, 91,
611-614 (2) Wikipedia- Atmospheric pressure
chemical ionization, https://en.wikipedia.org/wiki/Atmospheric-pressure_chemical_ionization,
accessed March 3, 2017.
Overview
In this lab, we will carry out a Wittig reaction (see
Klein, pp 965-967) to synthesize
1,4-diphenyl-1,3-butadiene from cinnamaldehyde (eq 1). In
this particular Wittig, the alpha protons of the
phosphonium salt are sufficiently acidic to be
deprotonated by aqueous NaOH, allowing the reaction to be
carried out in aqueous solution. This makes this method
much greener than the normal Wittig conditions of using
n-butyl lithium in ether or DMF solvent. It should be
noted, however, that the traditional Wittig reaction has
very poor atom economy, generating an 18-carbon side
product (Ph3PO), so it can hardly be though of
as "Green" unless this side product is recycled through a
reduction process.
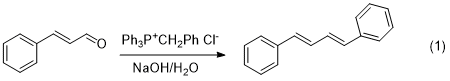
We will take a closer look at the reaction products and
completeness by carrying out thin-layer chromatography
coupled with mass spectrometry (TLC-MS). Thin-layer
chromatography is a powerful separation method often used
to follow reactions to completion and check product
purity. Please refer to Mohrig, Chap 17 for more back
ground info on TLC. The separated spots on our TLC plates
can be extracted by a suitable solvent and the extract
then introduced into a mass spectrometer that is suitable
for analyzing liquids. This requires a different kind of
mass spectrometer than the type that is most commonly used
in conjunction with gas chromatography (GC-MS).
Specifically, the MS we will use employs atmospheric
pressure chemical ionization (APCI), rather than the more
familiar electron-impact ionization (EI-MS). In APCI the
ionization of the molecules occurs via proton transfer
reactions to form "M+H" molecular ions. Therefore, unlike
in EI-MS, the highest m/z value in the spectrum
usually corresponds to M+H and is equal to the MW of the
compound plus one. Also, APCI is a much "softer"
ionization method than EI, meaning that little
fragmentation of the molecular ion is observed, making it
easier to be sure about the actual molecular weight of the
compound.
Procedures
Reaction:
Add 0.25 g cinnamaldehyde, 0.80 g benzyltriphenylphosphonium chloride (BTPC), and a magnetic stir bar to 10 mL Erlenmeyer or round-bottom flask. Carefully add 5.0 mL 10M NaOH and stir the suspension for 30 min at room temperature.
Work Up:
Collect the crude solid product by vacuum filtration washing with water until the filtrate is no longer basic to litmus. Allow the solid to dry on the Buchner funnel for 10 min and then determine the crude yield. Save a sample of the crude for mp and TLC analysis and recrystallize (Mohrig, chap 15) the rest from the minimum volume of ethanol. Determine the final yield after dryingon teh Buchner funnel for at least 10 min.
Analysis:
Obtain 1H NMR (CDCl3) and IR spectra of the recrystallized product. Do mp determinations on both the crude and recrystallized products.
TLC (Refer to Mohrig, Chapter 17, for details of how to carry out these procedures.)
- Prepare spotting solutions of (1) cinnamaldehyde, (2) BTPC, (3) the crude product and (4) the final product. Do so by dissolving 10 mg of each compound in 1.0 mL CH2Cl2.
- Obtain a TLC plate (silica gel with fluorescein indicator) and use a straight edged to lightly make a pencil line 1 cm from one end. Spot each solution at even intervals along the pencil line on the plate. Make sure to record in your notebook the order in which you make these spots.
- Develop the TLC plate in a TLC-jar using 1:9 ethyl acetate/heptane as the elution solvent.
- Visualize the plate under UV light outline all spots lightly with a pencil.
- Measure distances of all spots, calculate Rf values and carefully sketch the developed plate in your lab notebook.
TLC-MS - The instructor will assist you in obtaining APCI mass spectra of several of the spots on your TLC plate. The mass spectrometer is located in SLC 339.