Expt #1. Oxidation of 4-Methyl-2-pentanol
Relevant textbook reading - Brown and
Poon, Chapter 8.2F, Mohrig, Technique 11.1-11.4
Literature References - (a) Stevens,
R.V.,Chapman, K. T.,and Weller. H. N., J. Org.
Chem. 1980, 45,
2030-2032. (b) Mohrig,
J. R.; Nienhuis, D. M; Linck, C. F.; Van Zoeren, C.;
Fox, B. G.; Mahaffy, P. G. J. Chem. Educ.
1985, 62, 519-521.
Overview – 4-Methyl-2-pentanol will be oxidized
using hypochlorous acid (HOCl) (eq 1). The HOCl is
generated in situ from the reaction of acetic
acid with aqueous sodium hypochlorite (household bleach
or Clorox™). The product will be carefully distilled and
characterized by NMR, ASAP-MS, and refractive index.
Background - The method we are using
for alcohol oxidation is regarded as a relatively
"Green" method because it does not employ heavy metal
based oxidizing agents (e.g., Cr or Mn) nor does it
generate any toxic side products. It is often referred
to as the Chapman-Stevens oxidation in honor of the
scientists who first reported it.
The
mechanism has been proposed to start with a
proton transfer to the HOCl oxidant. The resulting H2OCl+
is attacked nucleophilically by the alcohol to generate
(after proton transfer) an ROCl intermediate, which then
undergoes an E2-like reaction to form the ketone.
In order for the oxidation to reach completion excess
HOCl must be used. However, the concentration of
hypochlorite in household bleach, often stated as 6.0%,
varies significantly. Therefore, the presence of excess
HOCl cannot be assured by simply using a predetermined
volume calculated based on the bleach concentration.
Instead, we will test for the presence of HOCl using
KI/starch test paper. This paper is impregnated with
iodide ions and starch and will turn black if treated
with an oxidizing agent (e.g. HOCl) which oxidizes the
iodide ions to iodine molecules (I2), which
in turn form a blue-black complex with the starch:
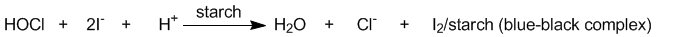
Pre-Lab - Make sure you
determine what product is expected and include it
(name, structure, and properties in your table of
chemicals. Also try to come up with a balanced
chemical equation for the reaction being performed.
Also make sure to calculate amounts of chemicals
needed in mL for liquids and g for solids and include
that in your table.
Procedures
Running the reaction.
-
Place 0.050 mol of
4-methyl-2-pentanol and a magnetic stir bar in an
Erlenmeyer flask.
- Carefully add 3.0 mL of glacial acetic acid to the
alcohol with stirring.
- Suspend a separatory funnel over top of the flask
using a metal ring.
- Add 67.5 mL of bleach (6.0% NaOCl) to the
separatory funnel. (Adjust the volume if the stated
concentration of the bleach is not 6.0%)
- Use the separatory funnel to add the bleach drop
by drop to the alcohol/acetic acid mixture over a
period of about 15 min. Monitor the temperature of
the solution during the addition and apply an ice
bath and/or slow the addition rate as necessary to
keep it in the range of 25-35 °C.
- When the addition of the bleach is complete, use a
KI/starch test strip to test the solution for excess
hypochlorous acid. If the test is negative then add
additional bleach. (See background above.)
- Stir the reaction mixture for an additional 30
min on a warm water bath at approx 35 °C, testing
with KI/starch paper every 5 min as above and adding
more bleach if necessary.
Work-up procedures.
- Destroy excess HOCl by adding 0.5 mL saturated
sodium bisulfite (NaHSO3). Test the
reaction for any remaining hypochlorous acid and, if
necessary, add additional sodium bisulfite.
- Add 5 drops of thymol blue (a pH indicator).
- Add enough 6M NaOH until the solution turns a
light blue (indicating a basic pH). (The approximate
volume of NaOH solution necessary for this step can
be calculated by simply equating the moles of NaOH
necessary to the moles of acetic acid started with.)
- Add 7.5 g solid NaCl and stir until it all
dissolves. (See the discussion of "salting out" in
Mohrig, chapter 11.)
- Decant the liquid into a separatory funnel and
extract with 10 mL diethyl ether (EtOEt) taking care
to vent frequently. (Review extraction procedures in
Mohrig, 11.1-11.4.)
- Transfer the organic layer to a labeled, dry
Erlenmeyer flask.
- Extract the aqueous layer two more times with 10
mL EtOEt each time combining the organic layer with
the original organic layer.
- Dry the organic extracts with Na2SO4
and decant the solution into a pre-weighed 50-mL
round bottom flask.
- Remove the ether solvent using the rotary
evaporator.
- Determine the yield.
Characterization
of Product
- Obtain the 1H NMR spectrum using CDCl3
as the solvent. One group will also be asked to
obtain the C-13 spectrum, which will be shared with
the rest of the class on Piazza.
Report
- Remember to report the yield, theoretical yield,
and percent yield and show the calculation of the
theoretical yield on an attachment.
- Presnt and discuss the mechanism of the reaction
as part of your discussion for this lab.
|