Expt 3 - Green Wittig Synthesis
Relevant textbook readings - Mohrig, Chapter 17, Klein, Chapter 19.10, pp 877-879.
Other References - Morsch,
L. A.; Deak, L.; Tiburzi, D.; Schuster, H.; Meyer, B. J.
Chem. Ed. 2014, 91, 611-614
Overview
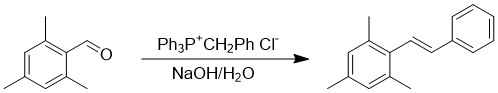
In this lab, we will carry out the Wittig reaction of
2,4,6-trimethylbenzaldehyde as shown in the equation below
and reported in the literature reference given above. When
using a benzylic phosphonium salt such as the one used
here, the alpha protons are sufficiently acidic (due to
extra resonance stabilization of the ylide) to be
deprotonated by aqueous NaOH, allowing the reaction to be
carried out in aqueous solution. This makes this method
much greener than the normal Wittig conditions of using
n-butyl lithium in ether or DMF solvent. It should be
noted, however, that the traditional Wittig reaction has
very poor atom economy, generating an 18-carbon side
product (Ph3PO), so it can hardly be though of
as "Green" unless this side product is recycled through a
reduction process.
We will check the reaction progress by carrying out
thin-layer chromatography (TLC). Thin-layer chromatography
is a useful separation method often used to follow
reactions to completion and check product purity. Please
refer to Mohrig, Chap 17 for more back ground info on TLC.
Procedures
Reaction:
Add 0.28 g of the aldehyde, 0.80 g benzyltriphenylphosphonium chloride (BTPC), and a magnetic stir bar to a 10-mL Erlenmeyer or round-bottom flask. Carefully add 5.0 mL 10M NaOH and begin stirring. After 15 min transfer approx 0.25 mL of the reaction mixture to a small test tube. Continue stirring for another 30 min. Meanwhile, to the test tube add 1 mL dichloromethane, shake the contents and transfer the lower DCM layer to another small test tube and dry it over sodium sulfate.
Work Up:
Collect the crude solid product by vacuum filtration washing with water until the filtrate is no longer basic to litmus. Allow the solid to dry on the Buchner funnel for 10 min and then determine the crude yield. Save a sample of the crude for mp and TLC analysis and recrystallize (Mohrig, chap 15) the rest from the minimum volume of ethanol. Determine the final yield after drying on the Buchner funnel for at least 10 min.
Analysis:
TLC. (Refer to Mohrig, Chapter 17, for more details on how to properly do these procedures.)
- Prepare spotting solutions of (1) the aldehyde reactant, (2) BTPC, (3) the crude product and (4) the final product. Do so by dissolving 10 mg of each compound in 1.0 mL CH2Cl2.
- Obtain a TLC plate (silica gel with fluorescein indicator) and use a straight edge to lightly make a pencil line 1 cm from one end. Spot each solution at even intervals along the pencil line on the plate. Make sure to record in your notebook the order in which you make these spots.
- Use the DCM extract from the 15 min reaction sample to make a 5th spot on the plate.
- Prepare a TLC development jar using 1:9 ethyl acetate/heptane as the elution solvent. Include a trimmed piece of filter paper stood up along one side (as shown in Mohrig) to help keep the atmosphere saturated with solvent fumes.
- Carefully place the spotted TLC plate in the development jar, cover it, and allow the plate to develop until the solvent has risen to within approx 1 cm of the top of the plate.
- Remove the plate and immediately mark the level that the solvent rose to.
- Allow a few minutes for all of the DCM to evaporate off of the plate then visualize it under UV light outline all spots lightly with a pencil.
- Measure distances of all spots, calculate Rf values and carefully sketch the developed plate in your lab notebook.
Obtain 1H NMR (CDCl3) and IR spectra of the recrystallized product.
Do mp determinations on both the crude and recrystallized products.