Expt 7. A
Photocatalyzed Intramolecular 2+2 Cycloaddition
Relevant textbook readings - Klein -
chapter 16.8 Mohrig
Literature References (1) Cookson, R. C.;
Crundwell, E.; Hill, R. R.; Hudec, J. J. Chem. Soc.
1964, 3062– 3075 (2) Krauch, C. H.; Metzner, W. Chemische
Berichte 1965, 98, 2106-2110.
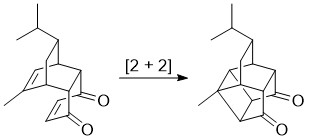
Overview - You will carry out an intramolecular
cycloaddition of the Diels-Alder from expt 6 to form
the cool cage compound,
1-methyl-10-isopropylpentacyclo[6.4.0.02,7.04,12.05,9]dodeca-3,6-dione.
Traditionally, this reaction requires a strong UV light
source and special quartz glassware. However, research
recently carried out at WSU has shown that the reaction
can be carried out with visible light using
4,4'-dichlorodibenzalacetone (DCDBA) as a photocatalyst.
Recall that DCDBA was prepared in expt 5. Also note that
the formation of the intramolecular 2+2 adduct provides
proof that the Diel-Alder adduct has the endo
stereochemistry because the exo adduct the C-C double
bonds are too far apart to be able to react with each
other.
The proton NMR and IR spectra will verify the reaction
product and a mp (lit mp = 92-94 ºC)2 will
also be obtained to verify purity.
Procedure
Reaction:
- Combine 50 mg of the Diels-Alder adduct with 12 mg 4,4’-dichlorodibenzalacetone in an NMR tube. Add 2 mL CH2Cl2 and cap and invert to dissolve. Place the tube in a test tube rack exposed to two 60-W LED lights for 1 hour.
Purification:
- Transfer the solution to a vial and evaporate the
solvent under N2 stream. Wash the obtained
crude solid with 0.5 mL toluene and then collect it by
vacuum filtration.
Analysis:
- Obtain the mp, proton NMR, and IR spectrum of the
product.