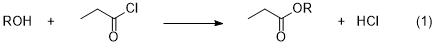Expt 3 - Preparation of a Propionate
Ester
Reading Assignment - Mohrig Chapter 19.1-4. Hart et. al. Chapter 10.9
Overview:
In this experiment you will react an assigned alcohol with propionyl chloride to form an ester (eq 1), which you will characterize by NMR, IR, and Mass Spectrometry.
Running the reaction.
To a small, dry round bottom flask add 11 mmol of propionyl chloride and 5 mL dichloromethane (DCM). Add a magnetic stirbar and a reflux condenser and cool the mixture on an ice bath. Add 10 mmol of the assigned alcohol dropwise via Pasteur pipet to the cold stirring solution of propionyl chloride. Heat the solution to a gentle reflux and stir for an additional 30 min to complete the reaction.
Work up the reaction.
Cautiously and slowly add 5 mL water to the stirring solution. The purpose is to hydrolyze any excess unreacted propionyl chloride remaining after the esterification is complete. Transfer to a small separatory funnel or large test tube suitable for liquid-liquid extraction.
Warning: be very careful to not confuse aqueous with organic layers in the procedures that follow. Remember you can always test each layer after separating by simply observing what happens when a few drops of water are added.
Transfer the organic layer to an Erlenmeyer flask. Extract the aqueous layer three times with 5 mL DCM, each time combining the organic extract with the first organic layer. Wash the combined organic extracts with 5 mL 1M NaOH followed by 5 mL DI water. Dry the organic solution over sodium sulfate.
Pass the dry solution through an approx 10-cm column of alumina to remove polar impurities, e.g. propionic acid. Wash the column through with another 20 mL dichloromethane. (This process of filtering an organic solution through a polar solid like silica or alumina is referred to as "flash chromatography" - see Chap 19.4 in Mohrig). Remove the solvent on the rotary evaporator and determine the yield of ester.
Analysis of Product
Obtain a proton NMR spectrum using chloroform-d as the solvent. A C-13 NMR spectrum may also be obtained if time allows. Also, obtain an IR spectrum. Turn in the product in a capped vial labeled with your name and lab section, product structure and name.