Expt #4. Radical
Polymerization of Styrene and Molecular Weight
Determination
Relevant textbook readings
- (1) Mohrig Chapter 7.1, 9.4. (2) Hart et. al.
Chapter 14.1-14.2
Literature Reference -
Wackerly, J. Wm.; Dunne, J. F J. Chem.
Educ. 2017 94, 1790-1793.
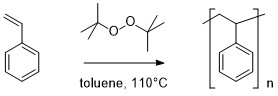
Overview - You will carry
out the radical polymerization of styrene (eq 1) and
obtain a 1H NMR spectrum of the resulting polystyrene.
Integration of the NMR spectrum will allow calculation
of the average molecular weight of the polymer.
Procedure
1. Place a
small piece of cotton in a Pasteur pipet and fill it
with alumina to a height of approx 3-4 cm. Use this
alumina column to filter 2 mL of styrene into a dry 5-mL
round-bottom flask (rbf) equipped with a magnetic
stirbar.
2. Add 2 mL
toluene followed by 300 mg di-tert-butyl peroxide
(DTBP).
3. Attach a
reflux condenser and reflux the mixture with stirring
for 1 h. (See Chapter 7.1 in Mohrig).
4. Cool to room
temperature. Use a pipette to add the reaction
solution to 30 mL methanol with rapid stirring.
5. Vacuum
filter (Mohrig technique 9.4) and transfer the obtained
solid to a pre-weighed watch glass. Place your labeled
watch glass in the oven (ca. 60 °C) for one week
to dry.
6. Determine
the yield of polystyrene and obtain the 1H
NMR spectrum in CDCl3.
Assigned questions.
1. Why was it
necessary to filter the styrene through alumina prior to
running this reaction? Hint: Look up styrene at
Sigma-Aldrich's website and check the specifications of
the product they sell.
2. Use the
integrations of the peaks at 6.2-6.7 (broad multiplet)
and 1.2 ppm (sharp singlet) to determine the average
molecular weight of your polymer. The instructor will
explain during the lab.
3. Write the
mechanism of the reaction including initiation and
termination steps.
4. Use the
mechanism to explain why polymer molecular weights are
not infinite.