Expt #4. Determination of Sterols in Dried
Mushrooms
Literature Reference - https://pubs.acs.org/doi/full/10.1021/jf104246z
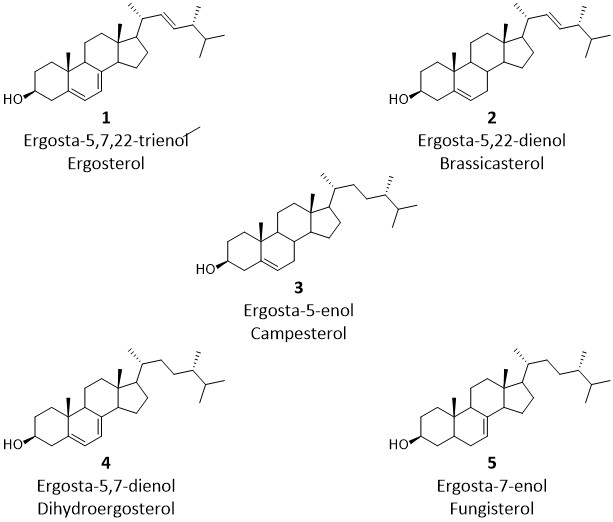
Overview – We will be using GC-MS to analyze the steroid alcohols (sterols - see below for some examples) present in different samples of dehydrated morel mushrooms.The sterols, which are in the form of fatty acid esters in the mushrooms, will be extracted using a Soxhlet extractor with petroleum ether and then saponified to remove the fatty acid groups. The free sterols will then be converted to trimethylsilyl (TMS) ethers before GC/MS analysis.
Procedures - An overview of the
procedures is shown in the figure at the bottom of the
page.
Extraction
Prep for GC/MS
- Obtain approx 4-5 g of dried mushrooms. Note the exact mass and number of specimens.Also note any additional information about the mushrooms being used.
- Break the mushrooms up with a mortar and pestle and then homogenize them more thoroughly in an electric coffee grinder.
- Save 0.5 g of the powder in a labeled clean vial.
- Transfer the rest of the powder into a pre-weighed extraction thimble. Get the exact mass of powder added.
- Add 10 mg of cholesteryl stearate to the powder. This compound is being used as a GC internal standard and will allow us to estimate the concentration of the sterols in the mushroom powder.
- Add 150 mL petroleum ether to a 250-mL rbf.
- Place the thimble in the well of a Soxhlet extractor. Fit the Soxhlet extractor to the rbf with the pet ether and attach a condenser on top. (Several Soxhlet extractors will be set up already in the lab but not enough for every team. Some groups will need to arrange times later in the week to run their extraction.)
- Run water through the condenser and heat the rbf on a heating mantle with a power setting of 60%.
- After 4 hours of running the extraction, turn off the heat and remove the rbf capping and storing it in your fume hood until the next step can be done.
- Use a rotary evaporator to remove all but a few mL of the pet ether. (If you inadvertently remove more than this then just add 3-4 mL of pet ether back to the flask.) (Most groups will need to arrange times outside of lab for this step because we only have one large rotovap available for our use.)
- Transfer the liquid to a 5-mL rbf and rotovap off the remaining pet ether.
- Add 3 mL 1.0 M NaOH/EtOH and a small stirbar. Attach a reflux condenser and reflux gently on a warm water bath (60 - 80 °C) for 1 h. (This is the saponification step.)
- Allow to cool and then transfer the solution to a test tube. Add 2 mL pet ether and 2 mL brine.
- Cap the test tube and shake the contents venting as necessary. Keep the top organic layer and re-extract the aqueous layer twice more with 2 mL pet ether.
- Dry the combined organic layers over sodium sulfate.
- Transfer to a clean, dry 10 mL rbf and rotovap off the pet ether.
- Add a stirbar, 0.80 mL pyridine, and 0.20 mL trimethylsilylimidazole (TSIM).
- Reflux gently (60 - 80 °C) for 1 h. (This step converts the alcohol groups to OTMS groups.)
- Transfer the solution to a GC/MS auto-injection vial, label it and turn it into the instructor.