(If the interactive diamond model doesn't work then here is a link to a jpeg image of the model.)
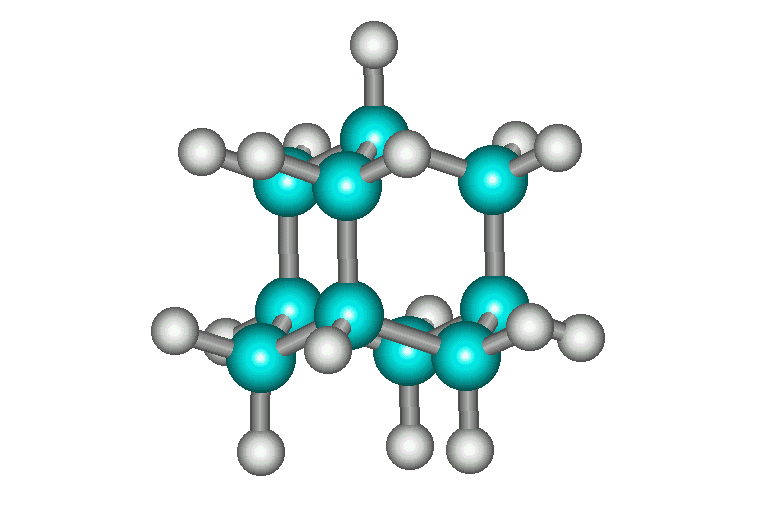
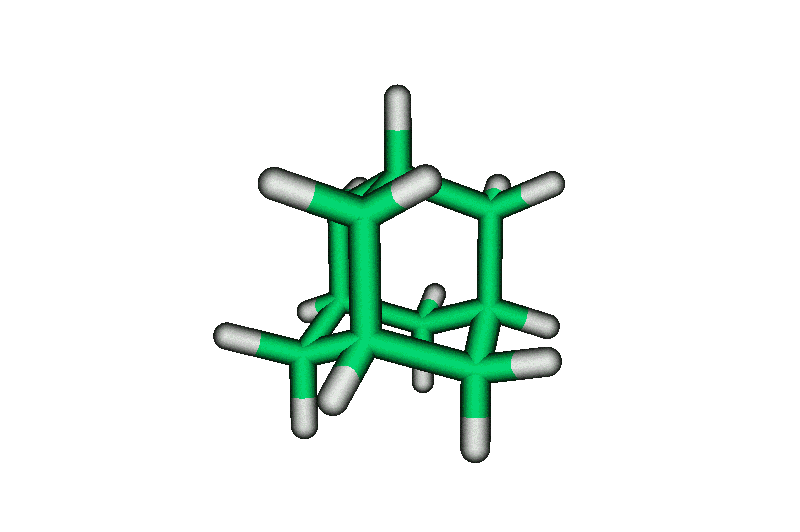
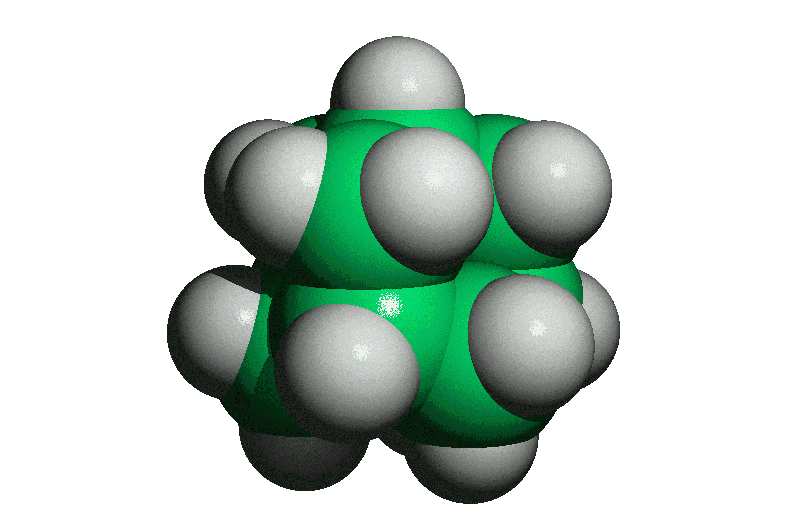
| Adamantane is the most stable isomer of formula, C10H16. It is named for its stability, "adamant" being a derivation from "diamond". Adamantane's stability is due partly to the fact it has no angle strain (all C's are perfectly tetrahedral, all bond angles are 109.5). Below are pictures of Ball and Stick, Cylinder, and Space-Filling models of adamantane. There are four cyclohexane chairs in the molecule - can you find them? | ||
 |
 |
 |
| In fact, diamond can be thought of being made up of
many adamantane units. Here is an interactive model of a
(extremely small!) diamond.
This requires the Hyperchem WebViewer available for free at http://www.hyper.com/products/Professional/WebViewer.htm)
(If the interactive diamond model doesn't work then here is a link to a jpeg image of the model.) |
||