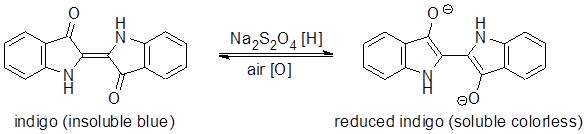Chemistry
351, Summer 2019, Winona State University, Dr. T. Nalli
|
Experiment #8. Organic Dyes
Overview
The process of dyeing is one of the earliest types of chemical process used by humans. The overall process involves both a colored compound (a pigment) and a mechanism for pigment adhesion to the fabric. In this lab you will dye various fabrics via two common adhesion mechanisms: physical entrapment and covalent bonding. Physical entrapment refers to cases where the dye is induced to form solid particles within the fabric which become entrapped within the network of fibers. The process historically used to dye blue jeans, indigo vat dyeing, is an example of a dye process that uses physical entrapment to adhere the dye molecules, which then can slowly fade away as the surface of the fabric is worn away. The more modern “fiber reactive” dyes use covalent bonding (usually through an SNAr mechanism) to adhere the dye molecules to hydroxyl-containing fabric molecules to form extremely brilliant and color-fast garments.
Part 1. Indigo Vat Dyeing.
In this process the deeply colored, insoluble indigo is reduced to a soluble, colorless form by sodium dithionite (Na2S2O4) (eq 1). The fabric is immersed in the colorless solution of reduced dye and then removed. Upon removal, the indigo air-oxidizes back to the insoluble form leaving blue particles trapped within the fibers
Procedure for Indigo Vat Dyeing
Prepare a solution of 1.5 g NaOH(s) in 5 mL
water in a 50-mL Erlenmeyer flask. Add 1.5 g indigo
and a magnetic stir bar to the solution. Add 20 mL
water to the stirring mixture. Heat the solution to
approx 50 °C and then add 5.0 g of Na2S2O4
in small portions. Keep stirring the solution at
approx 50 °C (use a hot water bath) for 20-30 min. At
this point the solution should be light yellow with no
sign of undissolved indigo powder. Add this solution
to 300 mL of warm (approx. 50 °C) water in a large
beaker. (If a blue or greenish tint develops then add
a few mg of additional dithionite.) Use this solution
to dye a cotton handkerchief.
Repeated immersions will work best to develop a darker
color. Hang the bandana up to dry in the back of the
fume hood.
Part 2. Tie-dyeing with Fiber-Reactive Dyes.
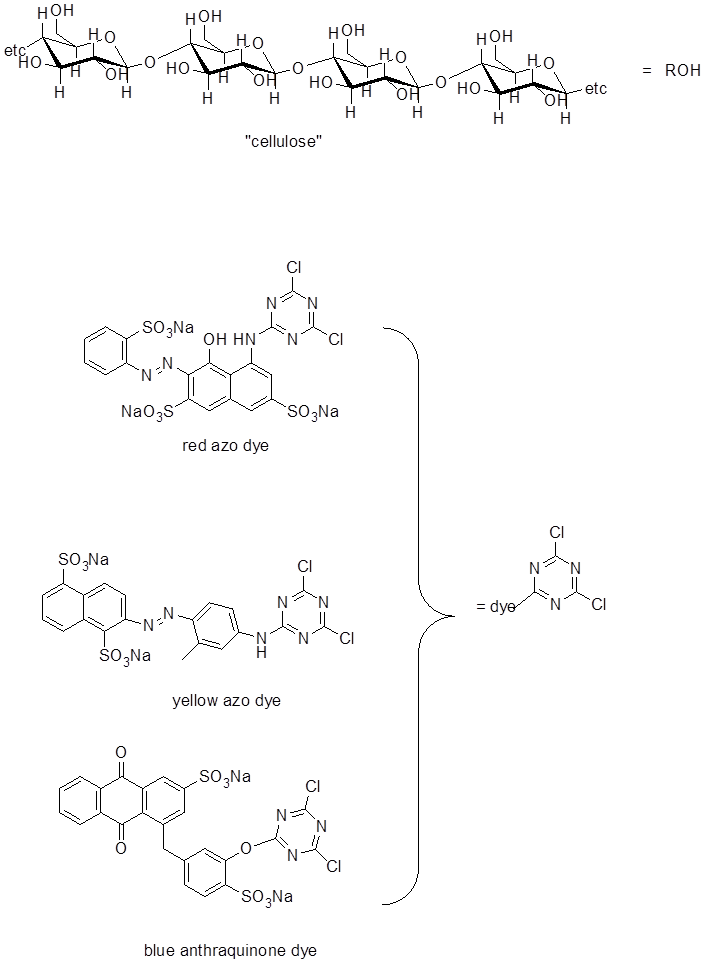
Fiber-reactive dyes adhere to cotton (essentially cellulose) through the formation of ether linkages to the OH groups of the cellulose. They consist of a highly-conjugated light-absorbing group (responsible for the color), which is linked to a dichlorotriazene group (Scheme 1). The cellulose is activated by treatment with base (Na2CO3) to form alkoxide groups which then undergo nucleophilic attack on the triazene rings and substitute for the chlorine leaving groups (Scheme 2). Both chlorines can be replaced and to the extent that this occurs the fibers of the fabric will be cross-linked, thus enhancing its durability. Procedure for Tie Dyeing
See https://prochemicalanddye.net/downloads/dl/file/id/111/product/0/tie_dye_using_pro_mx_directions.pdf. Most of steps 1-3 have been done for you.
Soak the t-shirt in soak solution (aqueous Na2CO3) for at least 10 min and then ring it out as described in step 2 before tying up and applying dye.
Make a dye solution to share with the rest of the class by dissolving one of the available Procion™ Dyes in 400 mL of 10% aqueous urea. See the table in step 4 of the above linked document for the approximate amount of dye powder to use.
Tie up the soaked t-shirt using rubber bands according to your creative desires. See https://prochemicalanddye.net/downloads/dl/file/id/112/product/0/tie_dye_folding_directions.pdf for ideas.
Use a 50-mL plastic syringe to apply dye solutions to the t-shirt. See step 5 of the linked procedure.
Place the t-shirt in the supplied plastic bag and bring it home and allow the dye to cure for at least 4 hours but preferably overnight.
Rinse the garment as described in step 6 and then wash it (if using a washing machine do it in a load by itself) using ordinary laundry detergent.
Scheme 1. Fiber-Reactive Dyes and Cellulose
Structures
Scheme 2. Reaction of Fiber-Reactive Dyes with Activated Cellulose and Water
|