Expt #3. Diels-Alder Reaction of α-Phellandrene with
p-Benzoquinone
Relevant textbook readings - Klein -
chapter 17.7. Mohrig - chapter 15.
Literature Reference - Alder, K.; Stein, G.; Pries, P.; Winckler, H. Ber. Dtsch. Chem. Ges. 1929, 2337-2372.
Prelab - Make sure you calculate the volume of phellandrene and the mass of p-benzoquinone to use and include both in your table of reactants and products. Also make sure to include the theoretical yield of the product.
Overview - You will carry out the Diels-Alder reaction of the terpene natural product, (R)-(–)-α-phellandrene, with p-benzoquinone. The resulting product could conceivably be any of four possible diastereomers, however, the configuration of C-5 of the phellandrene must be retained as shown in equation 1.
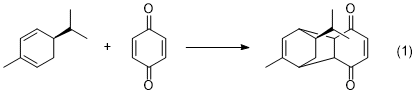
We will run the reaction and obtain a crystalline product
that will be thoroughly characterized by 1H, 13C,
DEPT, COSY, and HMQC NMR. An IR spectrum and mp (lit mp =
119 ºC) will also be obtained.
Procedure
Reaction:
- In a 10-mL round-bottom flask put 5 mmol p-benzoquinone and 5 mmol α-phellandrene.
- Add 4.0 mL of 1:1 EtOH/H2O and a boiling chip.
- Attach a reflux condenser, and heat at reflux for 30 min.
- Cool on an ice bath.
- Collect the crude product by vacuum filtration.
- Save a small sample of the crude product for a mp
determination.
Purification:
- Recrystallize the product from methanol. (Review chapter 15 in Mohrig).
- Get the crystals as dry as possible by sucking air through on the Buchner funnel for approx 15 min or by air drying for one week.
Analysis:
- Obtain the IR spectrum using the cast-film method of sample preparation. (see pp 286-287 in Mohrig).
- Obtain the mp of both the crude and recrystallized
product.
- Prepare a sample for NMR spectroscopy (CDCl3) and obtain a proton NMR. The group with the highest quality proton NMR (as judged by the instructor) will also obtain the following spectra: 13C NMR, DEPT-135, HMQC, HMBC, or COSY. The instructor will assist and the other groups will report on their own proton NMR in conjunction with the additional spectra from the chosen group.