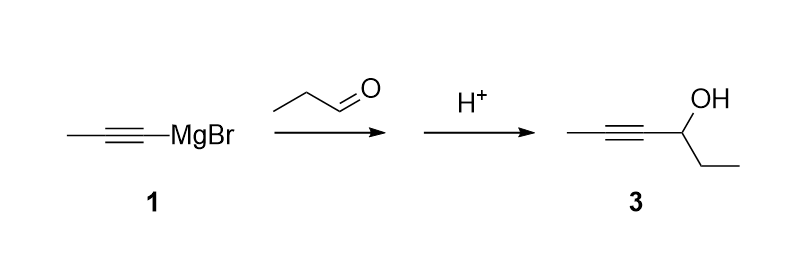
Expt #2. Grignard Synthesis of Hex-2-yn-4-ol
Relevant textbook readings – Karty, Chapter
18.4-18.5
Overview – We will react propynyl magnesium
bromide 1 with propanal 2. Acidic work up
should yield hex-2-yn-4-ol 3. The product will be
used as the starting material for expt #3.
Procedures
Running the reaction.
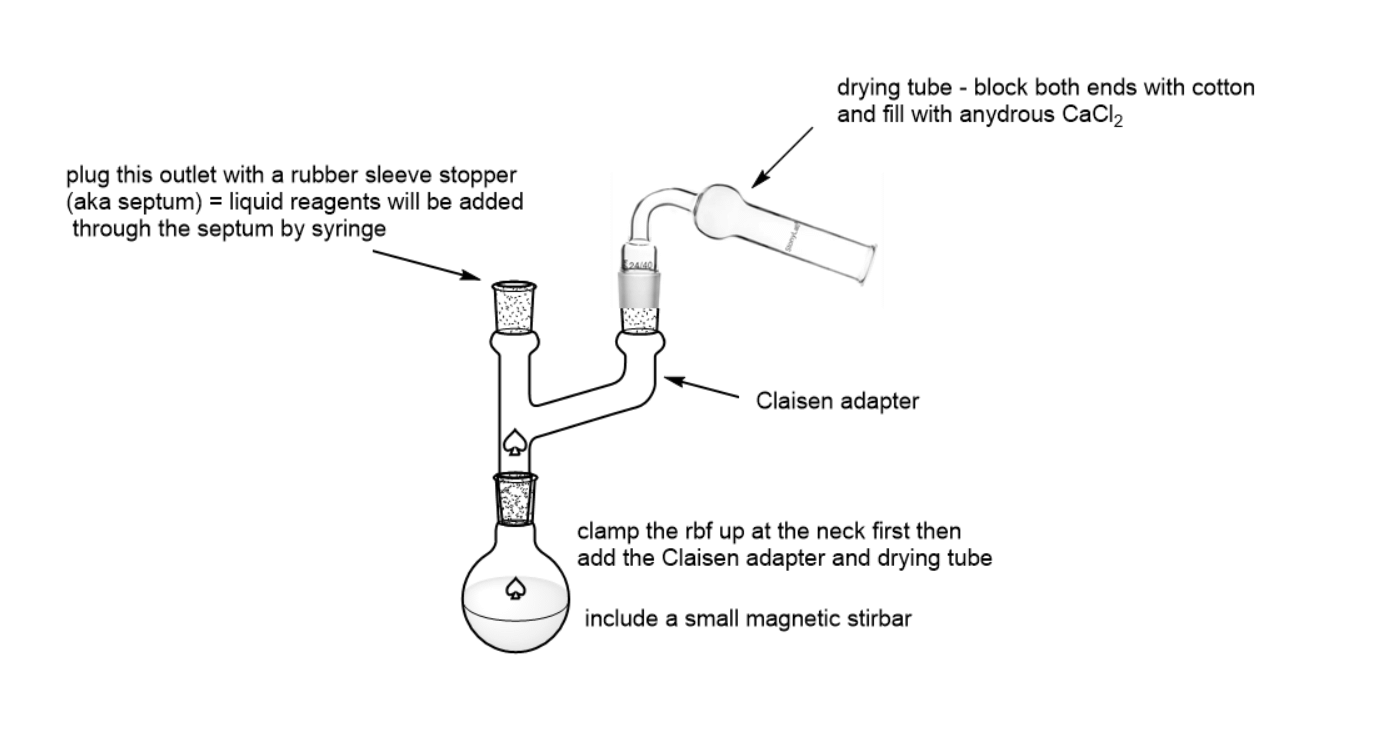
- Grignard reactions require dry glassware and anhydrous solvents. Take care to avoid water until the work up procedures are begun.
- Assemble the apparatus pictured below. The Claisen adapter functions to provide two entry ports to the reaction flask. The CaCl2 drying tube ensures no moisture from the air gets into the reaction. The liquid reagents are added by syringe through the rubber sleeve stopper.
Work-up procedures.
- Use an oven-dry 20-mL syringe to add 15 mL of 1 (0.5 M solution in THF).
- Cool the solution on an ice bath with stirring.Then to the stirring solution slowly (dropwise) add 0.55 mL propanal using a 1.0 mL plastic syringe.
- Allow the reaction to warm to room temperature and then continue stirring for 15 min at r. t.
- Slowly add 10 ml 1M HCl to the reaction solution. (At this point the drying tube can be removed and water does not need to be avoided anymore.)
- Transfer the solution to a separatory funnel and extract it 15 mLdiethyl ether. Repeat the extraction twice more.
- Transfer the combined ether extracts back into the sep funnel and wash with 45 mL saturated NaCl (aka "brine").
- Dry the ether layer over sodium sulfate for 10-15 min.
- Decant the ether into a dry pre-weighed 100 mL rbf and remove the solvent on the rotovap.
- Determine the yield and obtain a proton NMR and prepare a sample for GC-MS analysis.