Construction and Use of an Ion Selective Electrode
Read pages 591-606 in your textbook and answer questions 23-8 and 23-9 prior to coming to lab.
Introduction: Ion selective electrodes are the most frequently used analytical method to measure ion concentrations in solution. A pH electrode is the most frequently encountered ion selective electrode although ion selective electrodes for ammonium, fluoride, and cyanide are also frequently encountered. Commercially available electrodes such as pH electrodes are currently so compact that the user does not realize all the components that make up such an electrode. In this experiment you will construct an ion selective electrode for the measurement of the salicylate ion in solution. This will allow measurement of the active ingredient (acetylsalicylic acid) in common aspirin.
| Materials and Supplies | |
| Digital pH meter or Laptop computer with LabWorks Installed | Sodium chloride |
| Alligator clip leads | Polyvinyl chloride (PVC) |
| Reference electrode | Tetrahydrofuran (THF) |
| Screw cap vials with their bottoms removed | Sodium salicylate |
| Rubber septa | Silver wire, silver chloride |
| Tetrahexylammonium salicylate | Sodium tetraborate |
| Tetraoctylammonium salicylate | Aspirin tablets |
Preparation of the Salicylate Ion Selective Electrode (ISE)
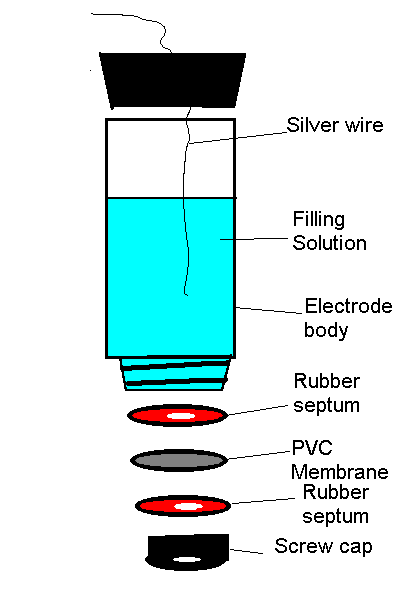
Prepare the ion selective membrane by dissolving 0.07g PVC and 0.15g of the tetraalkylammonium salicylate salt in 2.0mL of THF. Place the solution in an open 15mL beaker and let the solvent evaporate. When the solvent has evaporated (approximately 1 hour), remove the membrane with a forceps or glass spatula. It is possible to cut several disks of approximately 1cm diameter using a standard cork borer. Sandwich the membrane in between the septa as pictured below. Fill the electrode body with a solution of 0.01M NaCl and 0.10M sodium salicylate. Coat the silver wire with silver chloride by applying a positive potential relative to a platinum wire in an aqueous HCl acid solution. Repeat this procedure with the other tetraalkylammonium salicylate salt.

Verify the ISEs you just constructed work by preparing a calibration curve using standard sodium salicylate solutions. You will need to buffer your salicylate solutions with a 0.025M solution of Na2B4O7. This buffer is necessary to insure that the salicylate remains in the ionized form. Use concentrations of salicylate from 1M to 10-6M. Start your measurements at the lowest concentration to avoid contamination. A silver/ silver chloride electrode should be used as the reference electrode. Place your solution on a magnetic stirring plate. Prepare a calibration curve for both the tetrahexylammonium salicylate membrane and the tetraoctylammonium salicylate membrane. Measure the concentration of the salicylate ion in aspirin. The aspirin should be ground up, hydrolyzed in a measured volume of 1.0M NaOH for 15 minutes, adjusted with the measured volume of 1.0M HCl to near neutral pH, and then added to a measured volume of 0.025M Na2B4O7. Measure the ion concentration in a manner similar to that used to generate the calibration curves.
One of the major shortcomings of some ion selective electrodes is that they do not show enough selectivity to the ion of interest relative to other compounds of similar structure. Measure the selectivity of the ISE relative to 0.10M benzoate ion at a concentration of 10-4M salicylate. Do a similar selectivity measurement with two other anions of your choosing (you may decide to use an anion that was found in the aspirin tablet).
Report
Plot your calibration curves for both the tetraalkylammonium salicylate salts. Use a log concentration scale. Do you notice any differences for the two membrane types? What could cause such a difference?
Calculate the selectivity coefficient for benzoate and the two other anions that you used. Do you think these selectivity coefficients could be detrimental to your overall ion selective electrode efficiency? Why or why not? If so, what could you do to minimize such a problem?
Why was it necessary to buffer your salicylate solutions?
Draw a schematic of your overall measurement device.
If you created a LabWorks program to measure your cell potential, include a copy of the program in your notebook.
How did you coat the Ag wire? Why was it necessary to coat the Ag wire?
How does a fluoride ion selective electrode work?
What would you do differently if you were to do this experiment again?
Reference: Creager, Stephen E.; Lawrence, Kyle D.; Tibbets, Craig R. An Easily Constructed Salicylate-Ion-Selective Electrode for Use in the Instructional Laboratory. J. Chem. Educ. 1995 72 274.
 |
 |